Activation Energy and the Activation Complex
advertisement

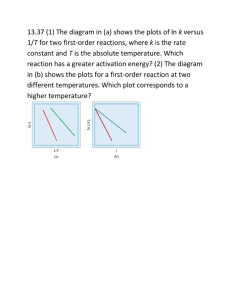
Group Three Activation Energy and the Activation Complex Another principle of the collision theory is related to the behaviour of the reactant particles as they collide. According to collision theory, when particles collide at the proper angle with the proper amount of energy, they form particles with structures unlike the structures of either the reactants or products. These intermediate particles are unstable and thus exist for very short periods of time. It is during this time the atoms rearrange themselves by producing a grouping of molecules considered to have bonds that are simultaneously beings formed and being broken. Each intermediate particle is called an activated complex. To illustrate the formation of an activated complex, consider the reaction between a molecule of hydrogen (H2) and a molecule of iodine (I2) to produce HI (Figure 1). For a reaction to occur, H2 and I2 must collide. If they approach from the wrong angle or have too little kinetic energy, they will rebound from each other without forming the product HI. If, however, the angle of approach is right and the particles have sufficient energy, an activated complex Figure 1 containing all for atoms (H2I2) is temporarily formed. Shortly after the activated complex is formed, it may break apart to re-form the original molecules of H2 and I2 or to form the product HI. The minimum amount of energy needed to form the activated complex is called the activation energy. Potential Energy Diagrams Why is there an activation energy for a chemical reaction to take place? Why don’t all collisions result in the formation of new products? To answer these questions consider the analogy illustrated in Figure 2. Imagine you are trying to roll a bowling ball up a very steep hill. On most tries the bowling ball slows down and stops before it gets to the top of the hill. The kinetic energy of the bowling ball is converted to potential energy as the ball slows down. Then it rolls back down on the same side of the hill. The hill acts as a barrier. Only occasionally does the bowler give the ball enough kinetic energy so that it gets to the top of the hill and rolls down the other side. Once one the downhill slope, the potential energy of the bowling ball gets converted back to kinetic energy, causing the ball to pick up speed. Figure 2 Picture a similar situation for molecules in a chemical reaction. During molecular collisions, atoms take up new bonding arrangements that have more potential energy than either the reactants or products. These atomic arrangements (i.e. the activated complex) have high potential energy like the bowling ball at the top of the hill. There is a minimum potential energy that must be achieved by colliding reactants before they can convert to some other form. This minimum potential energy is the activation energy for a given reaction. The relationship between the activation energy and the energy absorbed or given off in a reaction (i.e. and endothermic or exothermic reaction respectively) can be shown graphically on a potential energy diagram (Figure 3). Transition State Figure 3 It shows how the energy of the reacting system changes as the reaction proceeds. The potential energy of each chemical (reactants, the activated complex, and products) is indicated along the vertical axis. The progress of the reaction is plotted along the horizontal axis or reaction pathway. All potential energy diagrams have a similar profile. The reaction passes through energy maximum, known as the transition state. It is at this highest energy point that the activated complex is formed. The difference between the energy of the reactants in the beginning and when they are in the transition state is the activation energy or Ea. The activation energy, you will recall, is the minimum energy required for the reaction to occur and corresponds to the energy necessary to reach the transition state and form the activated complex. Most reactions have activation energies in the range of 100 to 200 KJ for every mole of reactant present. Figure 4a shows that if the products of the reaction are at a lower energy than the reactants, the reaction is exothermic. This type of reaction gives off energy, usually in the form of heat, and this indicated by a negative enthalpy (H) value. The change in enthalpy or H is the heat released or absorbed during a reaction, under constant pressure. If the products are of higher energy than the reactants (Figure 4b), we are dealing with an endothermic reaction. In either case the activation energy is always the difference between the energy of reactants initially and the highest point on the reaction pathway. Figure 4 (Exothermic) (Endothermic) The activation energy for a reverse reaction is not the same as the activation energy for its forward reaction. For example, nitrogen monoxide will react with ozone to produce nitrogen dioxide and oxygen: NO (g) + O3 (g) 2 NO2 (g) + O2 (g) For this reaction, the activation energy, Ea, is +10 KJ/mol, while the enthalpy change, H, is –200 KJ/mol (see Figure 5). We find that collisions between nitrogen dioxide and oxygen sometimes lead to the formation of nitrogen monoxide and ozone. 2 NO2 (g) + O2 (g) NO (g) + O3 (g) This reverse reaction will have an enthalpy change of +200 KJ/mol. As a result, the activation energy of the reverse reaction (E’a) will be 210 KJ/mol (10 KJ/mol + 200 KJ/mol). The activation energy of the reverse reaction is very high, indicating that the reaction will be very slow. Figure 5 Demonstration 1: Demonstration 1: Background: The wax of a candle burns readily in the presence of oxygen. - Place a candle on the demonstration table and ask students questions like: o Why is the candle, in the presence of oxygen, not reacting? o How can we get this reaction to occur? o What is the function of the match in causing the reaction to occur? Demonstration 2: - Create a way to demonstrate activation energies: a suggestion would be rolling a ball up a hill, very similar to the diagram under the potential energy section in these notes. Sample Questions 1. Define the terms ‘activation energy’ and ‘activated complex’. 2. Differentiate between activated complex and transition state. 3. What is a potential energy diagram? What does it show? 4. The potential energy diagram shown below is for the hypothetical reaction C A + B. (a) Is the reaction exothermic or endothermic? (b) Select the number on the diagram that indicates the (i) activation energy for the forward reaction (ii) activation energy for the reverse reaction (iii) potential energy of the reactant (iv) potential energy of the activated complex (v) the enthalpy of the reaction 5. In the hypothetical reaction, X + Y Z, the enthalpy of the forward reaction is H = -36 KJ/mol. the activation energy for the forward reaction is 73 KJ/mol (a) Draw a potential energy level diagram for this reaction. Be sure to label all the necessary parts. (b) What is the activation energy for the reverse reaction?