answers - handout on collision theory and activation energy
advertisement

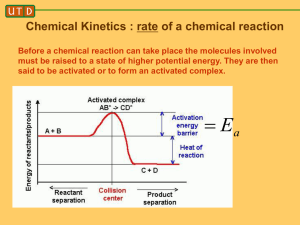
1 answers ­ collision theory handout..notebook March 10, 2015 1. Define“reaction rate”. The amount of reactant changing per unit of time. 2. a) The Collision theory states that particles (atoms, ions, molecules, etc…) must collide for a reaction to take place. b) Just colliding is not enough to guarantee a reaction. The particles must also collide with enough Kinetic energy. c) Particles must also collide with the right/proper Orientation (see Fig 18.4 pg. 543). d) Which part (A or B) of Fig 18.4 represents reactants colliding with proper orientation and making products? A Mar 2­11:57 AM 3. Copy Fig 18.5 on pg. 543. Refer to this graph to answer these questions: Video "Activ Energy" Mar 2­11:57 AM 1 1 answers ­ collision theory handout..notebook March 10, 2015 4. Define “activation energy”. The minimum energy colliding particles must have in order to react. 5. a) Define “activated complex”. An unstable arrangement of atoms that forms momentarily at the peak of the activation energy barrier. b)What two things are necessary for an activated complex to form? ­ sufficient energy ­ proper orientation c) Does the activated complex last very long? No d) What two things can happen after the activated complex forms? ­ products form ­ reactants re­form e) Which is more likely? Equal chance of either Mar 2­11:57 AM 6. a)What has higher energy? Reactants b) Is energy absorbed or released in progressing from reactants to the activated complex? Absorbed c) Once the activated complex is formed, will it always proceed to form products? No d) If a reaction requires a large amount of activation energy, is the reaction rate slow or fast? Slow Why? Not as many particles will have the necessary activation energy e) Will changing the temperature change the amount of activation energy to make products? No ... it only changes the number of particles with the necessary activation energy. 7. Draw a graph for an endothermic reaction. Energy Time Mar 2­11:57 AM 2