TG_Intro_to_Macromol_r7 - RI
advertisement

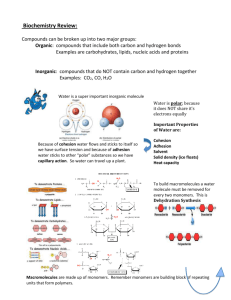
SAM Teachers Guide Introduction to Macromolecules Overview This activity introduces fundamental characteristics of biological macromolecules. It is a preliminary activity that supports the Lipids and Carbohydrates activity as well as the Proteins and Nucleic Acids activity. This is not a full activity, being only three pages, and is not meant to be used by itself. If you have the time and think the material in the Introduction to Macromolecules activity is pertinent to your course, we recommend that you set up a class where they do both the Introduction to Macromolecules and either the Lipids and Carbohydrates or the Proteins and Nucleic Acids together. Learning Objectives Students will be able to: Identify the four types of macromolecules and provide examples of their roles in living beings. Order relevant cellular objects by size, for example, cell, nucleus, macromolecule, molecule, atom. List four or five of the six elements that make up the macromolecules of living things. Identify characteristics common to biological macromolecules: that they can have hundreds or thousands of atoms, that they are made of six or fewer elements, that many are polymers made of closely related or identical units called monomers. Possible Student Pre/Misconceptions Students may have difficulty grasping the size of molecules relative to tangible objects. For example, they may think that two powers of ten indicate a twentyfold difference in size rather than a one-hundred-fold difference. Please use the feedback form for this activity to tell us of additional student misconceptions about macromolecules that come up during the activity. Models and Possible Discussion Questions Page 1 – The Four Families Model: Powers of Ten Run the model with the class, asking students to predict what they will see at each next higher or lower power. Point out the informative text that changes with each power of ten: o the description of the image, shown just above and to the left of the image o the current power of ten, shown just below and to the left of the image o the size of the image, shown just below and to the right of the image Point out the “Increase” and “Decrease” buttons (available when the model finishes running, or by clicking the “Manual” button) that allow students to control the pace and direction themselves. Possible Discussion Questions: What is the jump in size between each power of ten? What is the jump in size across two, three, etc., powers of ten? What instruments do scientists use to "see" at various powers of ten? Page 2 – “Life-size” Molecules Model: 3D Molecules If this is your students' first experience with 3D molecules, make sure they understand how to rotate the molecules by clicking and dragging with the mouse, and that they also know how to zoom in and out by holding the shift key down at the same time. Note that the selected small molecule changes size when each different large molecule is selected. The small molecule is re-sized in relation to the size of the atoms in the large molecule. For example, methane is smaller than a membrane lipid, but is very small compared to glycogen. Possible Discussion Questions: Why might shape be important for biological molecules? Why might size be important? Note that the final question on this page of the activity asks students to suggest some reasons that macromolecules have varying sizes and shapes. Students should not be expected to come up with a “correct answer” at this time. Instead, the intent is to encourage students to start considering why variation in size and shape might be important. Page 3 – Polymers and Monomers Model: Polymerization Reaction Chamber Emphasize that proteins, DNA, and carbohydrates are made of monomers, which are held together with covalent bonds. Note that one polymer has not been modeled here —one that branches. This type of polymer will be addressed in the polysaccharide section of the carbohydrates activity. Possible Discussion Questions: How are polymers built in nature, as opposed to in this model, the polymerization reaction chamber? Connections The Introduction to Macromolecules activity provides an optional introductory set of materials to be paired with either the Lipids and Carbohydrates activity or the Protein and Nucleic Acids activity, both of which it supports directly. This activity also supports the biology activities that delve further into macromolecules: Protein Structure and DNA to Proteins. These activities in turn are supported by earlier SAM physics and chemistry activities. Chemical Bonds explores the different types of bonds that result from different patterns of sharing electrons; and Intermolecular Attractions helps students to predict and understand what happens between molecules once these chemical bonds have formed. The Solubility activity helps students relate the properties of the molecules to the environment in which they are found. Activity Answer Guide Page 1: 1. The individual DNA strands, which are macromolecules, are seen most clearly at 10 to the power of (c) 2. How many powers of ten are there between the DNA strands and you? (Hint: First figure out which exponential level in the Powers of Ten series is closest to the size of humans). (8) Page 2: 4. It is important for biological molecules to have a great variety of sizes, shapes, and surfaces. Suggest some reasons that this is true. There are many functions that macromolecules carry out, from genes which carry inherited traits, to enzymes, to molecules that form the structures in a cell. In order to perform so many varied functions, a great many different kinds of molecules are needed. Page 3: 1. Take a snapshot of your polymer with identical monomers and drag it in to the box below. 1. Which elements can be found in all four types of macromolecules? (a) (b) (d) 2. Which of the following elements are found only in proteins? (e) 3. Which macromolecule is lopsided? One end has only carbon and hydrogen, while the other end includes nitrogen and oxygen. Place a snapshot here, and use the annotation tools to point out each end. 2. Take a snapshot of your polymer with different monomers and drag it into the box below. 3. What are the features of macromolecules that distinguish them from other molecules? Make sure to look at each page of this activity to include important characteristics in your answer. Macromolecules are large molecules. Most are polymers constructed from a set of smaller units called monomers. Macromolecules are built from only a few different kinds of atoms, including carbon, hydrogen and nitrogen.