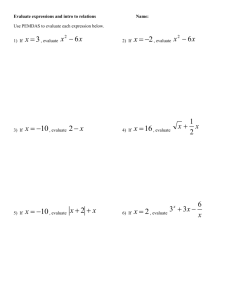Objectives
advertisement

Chemistry: Writing Chemical Equations Chapter 9 Unit Duration: Specific Resources/www sites Chemical reaction (rxn) Reactant Product Oxidation number Charge ions Coefficient Subscript Conservation of Matter Boot elements Diatomic Aqueous Liquid Ppt Direct combination Synthesis Decomposition Single replacement] Double replacement Combustiom Precipitate Polyatomic Cation Anion Weeks _____________________ Objectives: The student will be able to 9.1 The Nature of Chemical Reactions 1. Describe the characteristics of a chemical reaction. 2. Describe why cooking an egg portrays a chemical reaction. 3. Distinguish between reactants and products of chemical reactions. 4. Describe how oxidation numbers (charges) determine how an atom bonds to another atom. 9.2 Chemical Equations 5. Be able to write chemical reactions using words and symbols. 6. Be able to write balanced chemical reactions using the “inventory method”. 7. Be able to write balanced chemical reactions using the “divide oxygens then multiply all by 2 method” when there is an odd number of oxygens left to balance. 8. Explain how a balanced chemical equation illustrates the law of Conservation of Matter. 9. Be able to write “boot elements” in chemical reactions in diatomic form when they are by themselves. 10. Describe what a coefficient is when writing balanced chemical equations. 11. Describe what (l), (g), (s), (aq), (ppt) means in a written chemical equation and be able to label them in a reaction. 12. Be able to describe the difference between something written like: O2 and 2O. 9.3 Classifying Chemical Reactions 13. Name and be able to show examples of the five general types of reactions. 14. Be able to identify the 4 basic chemical reactions from general equations and chemical equations. Direct Combination (Synthesis) A + B AB Decomposition AB A + B Single-Replacement A + BX AX + B Double Replacement AX + BY AY + BX Combustion (carbon compound) + O2 Δ (heat) CO2 + H2O 15. Know how to determine if a precipitate is generated from a reaction. 16. Be able to identify proof of reactions. 17. Be able to identify polyatomic cations and anions 18. Be able to determin type of bond from Electronegativity and code for the bond type. 1 General Resource Materials Internet resources: http://www.phschool.com Text Book Chemistry Connections to Our Changing World —Prentice Hall--2000 Assessment Lab Guides/Reports Models (scoring rubrics) Quizzes, Unit Tests, Worksheets Notebook Quizzes Process Skills Take accurate measurements and convert Demonstrate safe lab practice and select appropriate equipment. Design models and evaluate their adequacy in representing chemical concepts. Collect data and make precise measurements. Organize, analyze, evaluate, make inferences, and predict trends from data. Communicate valid conclusions. Describe the connection between chemistry and future careers. 2 3