Chemical Reactions
advertisement

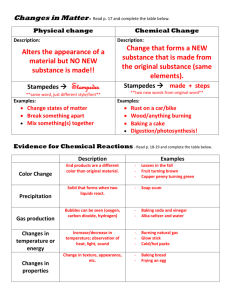
Balancing Equations Potassium Iodide and Lead Nitrate are mixed to form a precipitate (solid). After the reaction the mass of the flask will 1. 2. 3. Increase Decrease Stay the same 33% 1 33% 2 33% 3 Chemical Reactions Shown by chemical equations 4 Cu (s) coefficients + 1 O2 (g) 2 Cu2O (s) Symbols s l g aq yields, reacts to form reversible reaction heat is added solid liquid gas aqueous (dissolved in water) Sides? Reactant(s) Product(s) 2Fe + O2 2FeO “Rust” Why Balance? To show that the law of conservation of mass is satisfied To know the proportions the elements and compounds react in Balancing Equations using Atom Inventory Zn +2 HBr ZnBr2 + H2 Balanced!! 1 Zn 1 2 1 H 2 2 1 Br 2 Is everthing balanced? Leave Polyatomic ions together 2 AgNO3 + Na2S Ag2S + 2 NaNO3 Balanced!! 2 1 Ag 2 1 S 1 2 Na 12 NO3 12 2 1 Is everthing balanced? Never change subscripts Al2(SO4)3 + 6HCl 3H2SO4 +2 AlCl3 2 Al 12 Balanced!! 3 SO4 13 6 1 H 2 6 6 1 Cl 36 Is everthing balanced? Websites www.funbasedlearning.com (open with Internet Explorer) http://education.jlab.org/elementbalancing/inde x.html http://science.widener.edu/svb/tutorial/rxnbala ncingcsn7.html