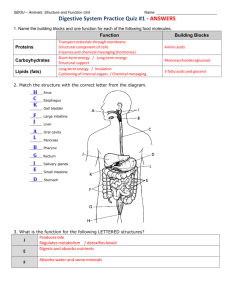
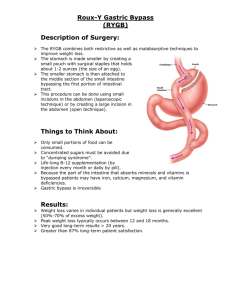
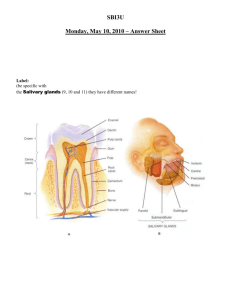
Fundamental Physiology and Anatomy of the Digestive
advertisement

Fundamental Physiology and Anatomy of the Digestive System Simply put, the digestive system is a portal for nutrients from the environment to gain access to the circulatory system. Before such transfer can occur, foodstuffs first have to be reduced to very simple molecules by a combination of mechanical and enzymatic degradation. The resulting sugars, amino acids, fatty acids and the like are then transported across the epithelium lining the intestine into blood. The focus of this section is to examine the "big picture" of digestive physiology and to look at fundamental aspects of digestive system structure. Overview of the Digestive System Consider for a moment a Big Mac. The purpose in your eating a Big Mac, other than simple hedonism, is to assimlate the nutrients it represents and make them available to build, repair and maintain your own tissues, as well as provide energy for studying and occasional other pursuits. You may have asked yourself - "Exactly what nutients are present in a Big Mac that I can assimilate?" MacDonald's comes close to full disclosure in this regard, but what they don't tell you is that in order to take advantage of these nutrients, you have to provide the means to carefully break them down into much smaller molecules that can be imported into blood. Luckily, your digestive system takes care of this very complex process so efficiently that most of the time you don't even need to think about it. At its simplest, the digestive system it is a tube running from mouth to anus. This tube is like an assembly line, or more properly, a dissembly line. Its chief goal is to break down huge macromolecules (proteins, fats and starch), which cannot be absorbed intact, into smaller molecules (amino acids, fatty acids and glucose) that can be absorbed across the wall of the tube, and into the circulatory system for dissemination around your body. The breakdown of foodstuffs like a Big Mac is accomplished through a combination of mechanical and enzymatic processes. To accomplish this breakdown, the digestive tube requires considerable assistance from accessory digestive organs such as the salivary glands, liver and pancreas, which dump their secretions into the tube. The name "accessory" should not be taken to mean dispensible; indeed, without pancreatic enzymes you would starve to death in short order. In many ways, the digestive system can be thought of as a well-run factory in which a large number of complex tasks are performed. The three fundamental processes that take place are: * Secretion: Delivery of enzymes, mucus, ions and the like into the lumen, and hormones into blood * Absorption: Transport of water, ions and nutrients from the lumen, across the epithelium and into blood * Motility: Contractions of smooth muscle in the wall of the tube that crush, mix and propel its contents Each part of the digestive tube performs at least some of these tasks, and different regions of the tube have unique and important specializations. Like any well-run factory, proper function of the digestive system requires robust control systems. Control systems must facilitate communication among different sections of the digestive tract (i.e. control on the factory floor), and between the digestive tract and the brain (i.e. between workers and managment). Control of digestive function is achieved through a combination of electrical and hormonal messages which originate either within the digestive system's own nervous and endocrine systems, as well as from the central nervous sytem and from endocrine organs such as the adrenal gland. Different parts of these systems are constantly talking to one another. The basic messages are along the lines of "I just received an extraordinary load of food, so I suggest you get prepared" (stomach to large intestine) or "For goodness sake, please slow down until I can catch up with what you've already given me" (small intestine to stomach). Finally, a note about differences in digestive anatomy and physiology among animals. The digestive systems of humans, dogs, mice, horses, kangaroos and great white sharks are, to a first approximation, virtually identical. If you look more carefully however, it becomes apparent that each of these species has evolved certain digestive specializations that have allowed it to adapt to a particular diet. These differences become particularly apparent when you compare a carnivore like a dog with a herbivore like a goat or a horse. Goats and horses evolved from ancestors that subsisted on plants and adapted parts of their digestive tracts into massive fermentation vats which enabled them efficiently utilize cellulose, the major carbohydrate of plants. In contrast, dogs evolved from animals that lived on the carcasses of other animals, and have digestive systems that reflect this history - extremely small fermentation vats and essentially no ability to utilize cellulose. Bridging the gap between carnivores and herbivores are omnivores like humans and pigs, whose digestive tracts attest to a historical diet that included both plants and animals. The image above shows a young omnivore in the company of herbivore and carnivore friends. Basic Functional Anatomy of the Digestive System -----------------------------------------------------------------------The digestive system is composed of the digestive or alimentary tube and accessory digestive organs. The basic terminology used to describe parts of the digestive system is shown below and more detailed description of each is presented in later sections. The digestive system depicted above - a carnivore - is the simplist among mammals. Other species, even humans, have a more or very much more extensive large intestine, and ruminants like cattle and sheep have a large set of forestomachs through which food passes before it reaches the stomach. Each of the organs shown above contributes to the digestive process in several unique ways. If you were to describe their most important or predominant function, and summarize shamelessly, the list would look something like this: * Mouth: Foodstuffs are broken down mechanically by chewing and saliva is added as a lubricant. * Esophagus: A simple conduit between the mouth and stomach - important but only marginally interesting. * Stomach: Where the real action begins - chemical digestion of proteins initiated and foodstuffs reduced to liquid form. * Liver: The center of metabolic activity in the body - its major role in the digestive process is to provide bile salts to the small intestine, which are critical for digestion and absorption of fats. * Pancreas: Important roles as both an endocrine and exocrine organ - provides a potent mixture of digestive enzymes to the small intestine which are critical for digestion of fats, carbohydrates and protein. * Small Intestine: The most exciting place to be in the entire digestive system - this is where the final stages of chemical digestion occur and where almost almost all nutrients are absorbed. * Large Intestine: Major differences among species in extent and importance - in all animals water is absorbed, bacterial fermentation takes place and feces are formed. In carnivores, that's about the extent of it, but in herbivores like the horse, the large intestine is huge and of critical importance for utilization of cellulose. -----------------------------------------------------------------------Microanatomy of the Digestive Tube -----------------------------------------------------------------------Remarkably diverse and specialized processes take place in different sections of the digestive tract, but there is a fundamental consistency in the architecture of the tubular digestive tract. From the mouth to the anus, the wall of the digestive tube is composed of four basic layers or tunics. * Tunica serosa is the outermost covering of the digestive tube. In most of the digestive tract (stomach and intestines) it consists of a thin layer of loose connective tissue covered by mesothelium (a type of squamous epithelium that lines body cavities); within the peritoneal cavity, this structure is also referred to as visceral peritoneum. In the abdominal cavity, the serosa on each side of the tube fuses together to form a suspensory structure called mesentery, which houses vascular and nervous supplies to the digestive tract and is continuous with the lining of the cavity. In regions outside of the abdominal cavity where the the digestive tube is essentially affixed to adjacent structures via its outer layer of connective tissue (esophagus and rectum), this tunic is referred to as tunica adventitia instead of tunica serosa. * Tunica muscularis endows the digestive tube with an ability to be motile. In most of the digestive tube, this tunic consists of two thick layers of smooth muscle. Muscle fibers in the inner layer are aligned circularly, whereas those in the outer layer have a longitudinal orientation. This combination of circular and longitudinal smooth muscle gives the tube an ability to perform complex movements that squeeze and propel ingesta in the lumen. Between the inner circular and outer longitudinal layers of smooth muscle is another critical component of the digestive tract's nervous system - the myenteric plexus. * Tunica submucosa, immediately beneath the mucosa, is a layer of loose to dense connective tissue containing blood and lymphatic vessels. The submucosa also contains the submucous plexus, a critical component of the digestive tract's nervous system which provides nervous control to the mucosa. * Tunica mucosa is the innermost layer of the digestive tube and lines the lumen. Among the four tunics, the mucosa is most variable in structure and function, endowing the tube with an ability to perform diverse and specialized digestive tasks along its length. Of critical importance in this regard are the epithelial cells that cover the mucosa and are thus in direct contact with the lumen. This epithelial cell sheet (lamina epithelialis) is distinctly different in different regions of the tract. Indeed, in most of the tract, several different cell types contribute to the epithelium, including cells dedicated to secretion, absorption or production of hormones. These distinctive differences in architecture of the epithelium can be seen below in the micrographs of mouse digestive tube. The magnification of all four images is identical and the epithelial layer is oriented toward the top. Beneath the epithelium, but still within the tunica mucosa is a layer - the lamina propria of loose connective tissue through which course blood vessels and lymphatics that supply the epithelium. This layer also contains lymphatic nodules important to immune functions of the digestive tract. Finally, beneath the lamina propropria is a thin layer of smooth muscle (lamina muscularis mucosae) which permits the mucosa to dynamically move and fold. -----------------------------------------------------------------------Like any other physiologic process, proper function of the digestive system requires robust control mechanisms. Maintaining adequate control requires intimate participation from both the nervous and endocrine systems, and many digestive diseases are associated with dysfunction in these relationships. Core concepts related to control of digestive function: * * * The Enteric Nervous System The Enteric Endocrine System Motility and Gastrointestinal Smooth Muscle Advanced and supplemental topics related to control of digestive function: * * * * The Gastrointestinal Immune System Microbial Life in the Digestive Tract Physiology of Peristalsis Electrophysiology of GI Smooth Muscle The nervous system exerts a profound influence on all digestive processes, namely motility, ion transport associated with secretion and absorption, and gastrointestinal blood flow. Some of this control emanates from connections between the digestive system and central nervous system, but just as importantly, the digestive system is endowed with its own, local nervous system referred to as the enteric or intrinsic nervous system. The magnitude and complexity of the enteric nervous system is immense - it contains as many neurons as the spinal cord. The principal components of the enteric nervous system are two networks or plexuses of neurons, both of which are embedded in the wall of the digestive tract and extend from esophagus to anus: * The myenteric plexus is located between the longitudinal and circular layers of muscle in the tunica muscularis and, appropriately, exerts control primarily over digestive tract motility. * The submucous plexus, as its name implies, is buried in the submucosa. Its principal role is in sensing the environment within the lumen, regulating gastrointestinal blood flow and controlling epithelial cell function. In regions where these functions are minimal, such as the esophagus, the submucous plexus is sparse and may actually be missing in sections. The image below shows part of the myenteric plexus in a section of cat duodenum. Pass your mouse cursor over the image to outline several enteric neurons. Pictuare7 In addition to the two major enteric nerve plexuses, there are minor plexuses beneath the serosa, within the circular smooth muscle and in the mucosa. Within enteric plexuses are three types of neurons, most of which are multipolar: * Sensory neurons receive information from sensory receptors in the mucosa and muscle. At least five different sensory receptors have been identified in the mucosa, which respond to mechanical, thermal, osmotic and chemical stimuli. Chemoreceptors sensitive to acid, glucose and amino acids have been demonstrated which, in essence, allows "tasting" of lumenal contents. Sensory receptors in muscle respond to stretch and tension. Collectively, enteric sensory neurons compile a comprehensive battery of information on gut contents and the state of the gastrointestinal wall. * Motor neurons within the enteric plexuses control gastrointestinal motility and secretion, and possibly absorption. In performing these functions, motor neurons act directly on a large number of effector cells, including smooth muscle, secretory cells (chief, parietal, mucous, enterocytes, pancreatic exocrine cells) and gastrointestinal endocrine cells. * Interneurons are largely responsible for integrating information from sensory neurons and providing it to ("programming") enteric motor neurons. Enteric neurons secrete an intimidating array of neurotransmitters. One major neurotransmitter produced by enteric neurons is acetylcholine. In general, neurons that secrete acetylcholine are excitatory, stimulating smooth muscle contraction, increases in intestinal secretions, release of enteric hormones and dilation of blood vessels. Norepinephrine is also used extensively for neurotransmission in the gastrointestinal tract, but it derives from extrinsic sympathetic neurons; the effect of norepinephrine is almost always inhibitory and opposite that of acetylcholine. The enteric nervous system can and does function autonomously, but normal digestive function requires communication links between this intrinsic system and the central nervous system. These links take the form of parasympathetic and sympathetic fibers that connect either the central and enteric nervous systems or connect the central nervous system directly with the digestive tract. Through these cross connections, the gut can provide sensory information to the CNS, and the CNS can affect gastrointestinal function. Connection to the central nervous system also means that signals from outside of the digestive system can be relayed to the digestive system: for instance, the sight of appealing food stimulates secretion in the stomach. In general, sympathetic stimulation causes inhibition of gastrointestinal secretion and motor activity, and contraction of gastrointestinal sphincters and blood vessels. Conversely, parasympathetic stimuli typically stimulate these digestive activities. Some of the prominent communiques enabled by nervous interconnections within the digestive tract have been named as reflexes and serve to illustrate a robust system of control. Examples include the gastrocolic reflex, where distention of the stomach stimulates evacuation of the colon, and the enterogastric reflex, in which distention and irritation of the small intestine results in suppression of secretion and motor activity in the stomach. Congenital and acquired derangements in the structure or function of the enteric nervous system are well recognized as causes of digestive tract disease. Examples include small intestinal motility disorders, gastric outlet obstructions and megacolon. The Enteric Endocrine System -----------------------------------------------------------------------The second of the two systems that control digestive function is the endocrine system, which regulates function by secreting hormones. Recall that hormones are chemical messengers secreted into blood that modify the physiology of target cells. A target cell for a particular hormone is a cell that has receptors for that hormone and can thus respond to it. Digestive function is affected by hormones produced in many endocrine glands, but the most profound control is exerted by hormones produced within the gastrointestinal tract. The gastrointestinal tract is the largest endocrine organ in the body and the endocrine cells within it are referred to collectively as the enteric endocrine system. Three of the best-studied enteric hormones are: * Gastrin: Secreted from the stomach and plays an important role in control of gastric acid secretion. * Cholecystokinin: A small intestinal hormone that stimulates secretion of pancreatic enzymes and bile. * Secretin: Another hormone secreted from small intestinal epithelial cells; stimulates secretion of a bicarbonate-rich fluids from the pancreas and liver. In contrast to endocrine glands like the anterior pituitary gland, in which essentially all cells produce hormones, the enteric endocrine system is diffuse: single hormone-secreting cells are scattered among other types of epithelial cells in the mucosa of the stomach and small intestine. For example, most of the epithelial cells in the stomach are dedicated to secreting mucus, hydrochloric acid or a proenzyme called pepsinogen into the lumen of the stomach. Scattered among these secretory epithelial cells are G cells, which are endocrine cells that synthesize and secrete the hormone gastrin. Being a hormone, gastrin is secreted into blood, not into the lumen of the stomach. Similarly, other hormones produced by the enteric endocrine system are synthesized and secreted by cells within the epithelium of the small intestine. Like all endocrine cells, cells in enteric endocrine system do not simply secrete their hormone continuously, which would not be very useful as a control system. Rather, they secrete hormones in response to fairly specific stimuli and stop secreting their hormone when those stimuli are no longer present. What stimulates the endocrinocytes in the enteric endocrine system? As you might deduce, in most cases these endocrine cells respond to changes in the environment within the lumen of the digestive tube. Because these cells are part of the epithelium, their apical border is in contact with the contents of the lumen, which allows them to continually "taste" or sample the lumenal environment and respond appropriately. To illustrate how control is implemented through the enteric endocrine system, consider the important example of preventing stomach acid from burning the epithelium of the small intestine: * Acid-laden ingesta flows out of the stomach, into the small intestine. * Acid in the small intestine stimulates secretion of the hormone secretin from endocrine cells in the intestinal epithelium. * Secretin stimulates the pancreas to dump a bicarbonate-rich fluid into the lumen of the intestine. * The bicarbonate neutralizes acid, which removes the stimulus for secretion of additional secretion. In addition to the hormones listed above, cells in the gastrointestinal tract also secrete a large battery of other peptide regulators that appear to act as paracrine agents or neurotransmitters, affecting such processes as motility, blood flow and growth of the digestive tract. Further discussion of the physiologic roles of enteric hormones is included in subsequent sections describing digestive organs. Additionally, more detailed descriptions of GI hormones, their receptors and mechanisms of action are to be found in the section on Gastrointestinal Hormones in the Endocrine System text. Gastrointestinal Motility and Smooth Muscle -----------------------------------------------------------------------Early in life, children notice that strange gurgling sounds sporadically emanate from their "stomachs," particularly in periods between meals. This simple observation reflects that fact that the digestive tube is quite muscular and that muscle contractions and motility are integral parts of digestive function. It follows that derangements in gastrointestinal motility can cause or result from digestive tract disease and that drugs which alter gastrointestinal motility affect digestive function. Two fundamental patterns of motility are conducted by the digestive tube: * Propulsion: foodstuffs must be propelled along the length of the digestive tube in order to be subjected to the sequential series of processing involved in dissassembly and absorption. The principal type of propulsive motility, seen particularly in the esophagus and small intestine, is peristalsis - a ring of muscle contraction appears on the oral side of a bolus of ingesta and moves toward the anus, propelling the contents of the lumen in that direction; as the ring moves, the muscle on the other side of the distended area relaxes, facilitating smooth passage of the bolus. Peristalsis * Mixing: If ingested materials were simply propelled through the digestive tube, you would expect very poor digestion and absorption, because the digestive enzymes would not be adequately mixed with the ingesta and the bulk of the ingesta would not come in contact with the epithelial cells that absorb nutrients. Segmentation contractions are a common type of mixing motility seen especially in the small intestine - segmental rings of contraction chop and mix the ingesta. Alternating contraction and relaxation of the longitudinal muscle in the wall of the gut also provides effective mixing of its contents. Segmentation Contractions Except for the first section of the esophagus, all the the muscle in the wall of the digestive tube is smooth muscle. Indeed, the patterns of motility seen in the gut are characteristic of smooth muscle, which has properties distinctly different from skeletal muscle. Smooth muscle fibers are arranged in intertwined, rather indistinct bundles, aligned in most areas of the tube in circular and longitutinal layers. Individual smooth muscle fibers are connected to neighboring smooth muscle cells by gap junctions, which allow these cells to be electrically coupled. The important consequence of this electrical coupling is that when an area of smooth muscle becomes depolarized, that depolarization spreads outward through adjacent sections of smooth muscle - this results in a wellcoordinated contraction of, for example, an entire ring of circular smooth muscle. Without electrical coupling through gap junctions, one would imagine that you would see contraction only of patches of circular or longitudinal muscle, which would have little effect on propulsion or mixing of ingesta. The Gastrointestinal Immune System -----------------------------------------------------------------------The lumen of the gastrointestinal tract is outside of the body and much of it is heavily populated with potentially pathogenic microorganisms. It is thus important that the immune system establish and maintain a strong presence at this mucosal boundary, and indeed, the digestive tube is heavily laden with lymphocytes, macrophages and other cells that participate in immune responses. Aside from all of its other functions, the gastrointestinal tract is a lymphoid organ, and the lymphoid tissue within it is collectively referred to as the gut-associated lymphoid tissue or GALT. The number of lymphocytes in the GALT is roughly equivalent to those in the spleen, and, based on location, these cells are distributed in three basic populations: Picture 8 * Peyer's Patches: These are lymphoid follicles similar in many ways to lymph nodes, located in the mucosa and extending into the submucosa of the small intestine, especially the ileum. In adults, B lymphocytes predominate in Peyer's patches. Smaller lymphoid nodules can be found throughout the intestinal tract. In the image of canine ileum to the right, three lymphoid follicles of a Peyer's patch can be seen. The muscularis is at the top left, and mucosal epithelium in the bottom right. * Lamina propria lymphocytes: These are lymphocytes scattered in the lamina propria of the mucosa. A majority of these cells are IgA-secreting B cells. * Intraepithelial lymphocytes: These are lymphcytes that are positioned in the basolateral spaces between lumenal epithelial cells, beneath the tight junctions (they are "inside" the epithelium, but not inside epithelial cells as the name may incorrectly suggest). Another important component of the GI immune system is the M or microfold cell. M cells are a specific cell type in the intestinal epithelium over lymphoid follicles that endocytose a variety of protein and peptide antigens. Instead of digesting these proteins, M cells transport them into the underlying tissue, where they are taken up by macrophages. Macrophages that receive antigens from M cells present them to T cells in the GALT, leading ultimately to appearance of immunoglobulin A-secreting plasma cells in the mucosa. The secretory IgA is transported through the epithelial cells into the lumen, where, for example, it interferes with adhesion and invasion of bacteria. T cells exposed to antigen in Peyer's patches also migrate into the lamina propria and the epithelium, where they mature to cytotoxic T cells, providing another mechanism for containing microbial assaults. In addition to the GALT discussed above, lymph nodes that receive lymph draining from the gut (mesenteric nodes) and Kupffer cells (phagocytic cells in the liver) play important roles in protecting the body against invasion. -----------------------------------------------------------------------Back to the index of Control of Digestive System Function Microbial Life in the Digestive Tract -----------------------------------------------------------------------The gastrointestinal tract contains an immensely complex ecology of microorganisms. Within the colon, a typical person harbors more than 400 distinct species of bacteria. The number of bacteria in the gastrointestinal tract vary dramatically by region. In healthy individuals the stomach and proximal small intestine contain few microorganisms, largely a result of the bacteriocidal activity of gastric acid; those that are present are aerobes and facultative anaerobes. One interesting testimony to the ability of gastric acid to suppress bacterial populations is seen in patients with achlorhydria, a genetic condition which prevents secretion of gastric acid. Such patients, which are otherwise healthy, may have as many as 10,000 to 100,000,000 microorganisms per ml of stomach contents. In sharp contrast to the stomach and small intestine, the contents of the colon literally teem with bacteria, predominantly strict anaerobes (bacteria that survive only in environments virtually devoid of oxygen). Between these two extremes is a transitional zone, usually in the ileum, where moderate numbers of both aerobic and anaerobic bacteria are found. Microbial Populations in the Digestive Tract of Normal Humans Stomach Jejunum Ileum Colon Viable bacteria per gram 0 - 103 0 - 104 105 - 108 1010 - 1012 pH 3.0 6.0-7.0 >7.5 6.8-7.3 The gastrointestinal tract is sterile at birth, but colonization typically begins within a few hours of birth, starting in the small intestine and progressing caudally over a period of several days. In most circumstances, a "mature" microbial flora is established by 3 to 4 weeks of age. It is also clear that microbial populations exert a profound effect on structure and function of the digestive tract. For example: * The morphology of the intestine of germ-free animals differs considerably from normal animals - villi of the small intestine are remarkably regular, the rate of epithelial cell renew is reduced and, as one would expect, the number and size of Peyer's patches is reduced. * The cecum of germ-free rats is roughly 10 times the size of that in a conventional rat. * Bacteria in the intestinal lumen metabolize a variety of sterols and steroids. For example, bacteria convert the bile salt cholic acid to deoxycholic acid. Small intestinal bacteria also have a important role in sex steroid metabolism. Finally, bacterial populations in the large intestine digest carbohydrates, proteins and lipids that escape digestion and absorption in small intestine. This fermentation is of relatively little consequence to species like humans and carnivores (except when it leads to flatulence). However, microbial fermentation, particularly of cellulose, is of critical importance to herbivores. Physiology of Peristalsis -----------------------------------------------------------------------Peristalsis is a distinctive pattern of smooth muscle contractions that propels foodstuffs distally through the esophagus and intestines. It was first described by Bayliss and Starling (J Physio (Lond) 24:99-143, 1899) as a type of motility in which there is contraction above and relaxation below a segment being stimulated. Peristalsis is not affected to any degree by vagotomy or sympathetectomy, indicating its mediation by the intestine's local, intrinsic nervous system. Peristalsis is a manifestation of two major reflexes within the enteric nervous system that are stimulated by a bolus of foodstuff in the lumen. Mechanical distension and perhaps mucosal irritation stimulate afferent enteric neurons. These sensory neurons synapse with two sets of cholinergic interneurons, which lead to two distinct effects: * One group of interneurons activates excitatory motor neurons above the bolus these neurons, which contain acetylcholine and substance P, stimulate contraction of smooth muscle above the bolus. * Another group of interneurons activates inhibitory motor neurons that stimulate relaxation of smooth muscle below the bolus. These inhibitor neurons appear to use nitric oxide, vasoactive intestinal peptide and ATP as neurotransmitters. -----------------------------------------------------------------------Last updated on October 7, 1995 Electrophysiology of Gastrointestinal Smooth Muscle -----------------------------------------------------------------------Normal gastrointestinal motility results from coordinated contractions of smooth muscle, which in turn derive from two basic patterns of electrical activity across the membranes of smooth muscle cells - slow waves and spike potentials. Like other excitable cells, gastrointestinal smooth muscle cells maintain a electrical potential difference across their membranes. The resting membrane potential of smooth muscle cells is between -50 and -60 mV. In contrast to nerves and other types of muscle cells, the membrane potential of smooth muscle cells fluctuates spontaneously. Because the cells are electrically coupled, these fluctuations in membrane potential spread to adjacent sections of muscle, resulting in what are called "slow waves" - waves of partial depolarization in smooth muscle that sweep along the digestive tube for long distances. These partial depolarizations are equivalent to fluctuations in membrane potential of 5 to 15 mV. The frequency of slow waves depends on the section of the digestive tube - in the small intestine, they occur 10 to 20 times per minute and in the stomach and large intestine 3 to 8 times per minute. Slow wave activity appears to be a property intrinsic to smooth muscle and not dependent on nervous stimuli. Importantly, slow waves are not action potentials and by themselves do not elicit contractions. Rather, they coordinate or synchronize muscle contractions in the gut by controlling the appearance of a second type of depolarization event - "spike potentials" which occur only at the crests of slow waves. Spike potentials are true action potentials that elicit muscle contraction. They result when a slow wave passes over an area of smooth muscle that has been primed by exposure to neurotransmitters released in their vicinity by neurons of the enteric nervous system. The neurotransmitters are released in response to a variety of local stimuli, including distension of the wall of the digestive tube and serve to "sensitize" the muscle by making its resting membrane potential more positive. Click on the graph to show (or hide) the muscle tension trace One can now step back and understand how a particular pattern of motility is achieved. Think for a moment about what happens when a large bolus of ingested food enters the small intestine: * The bolus distends the gut, stretching its walls. * Stretching stimulates nerves in the wall of the gut to release neurotransmitters into smooth muscle at the site of distension - the membrane potential of that section of muscle becomes "more depolarized." * When a slow wave passes over this area of sensitized smooth muscle, spike potentials form and contraction results. * The contraction moves around and along the gut in the coordinated manner because the muscle cells are electrically coupled through gap junctions. -----------------------------------------------------------------------Pregastric Digestion -----------------------------------------------------------------------Around the teeth and through the gums - look out stomach, here it comes. An animal's or person's state of health is only as good as its nutritional state, and nutrient intake depends on normal pregastric function. Maintaining an adequate intake of nutrients is often hampered by diseases affecting pregastric function, and a solid understanding of pregastric function is necessary not only to correct diseases affecting those systems, but also to insure nutritional support in the face of all diseases. As important as inadequate food intake is, the most prevalent nutritional disease of humans and pets in developed countries is obesity, and remarkable progress has been made in recent years to explain some of the long-standing mysteries about how we control how much we eat. Core concepts in pregastric digestion are presented as the following topics: * * * * * Control of Food Intake Physiology of Taste Salivary Glands and Saliva Prehension, Mastication and Swallowing The Esophagus Advanced and supplemental topics related to pregastric physiology: * * * * * Genetics of Food Intake, Body Weight and Obesity Taste Preferences Teeth and Mastication Dental Anatomy Chewing: All In a Day's Work Control of Food Intake and Body Weight ------------------------------------------------------------------------ The body is in a continual state of hunger, which is intermittently relieved by eating. This perpetual drive to eat is periodically suppressed by inhibitory impulses generated by such things as the presence of food in the gastrointestinal tract, the flow of nutrients into blood and other factors. After these "satiety factors" have dissipated, the desire to eat returns. Why is it important to understand the factors that control food intake? At least two major areas of import come to mind: * Obesity is the most prevalent nutritional disease of humans, dogs and cats in affluent societies such as ours, exceeding by far the number of nutritional deficiency diseases. * Metabolic demands of people and animals increase with sickness or trauma, often in conjunction with anorexia. Sickness combined with anorexia leads to accelerated starvation. Before going on, take a minute to reflect on observations you have already made about food intake, body weight and similar topics. You may have noticed, for instance, that: * * * Most animals as adults maintain a remarkably constant body weight. When it's cold, animals and people eat more than when it's hot. Children maintain energy balance with wildly varying intakes of food per meal. These kinds of observations suggest a very complex system in charge of regulating energy balance and body weight. What is known about control of food intake is often discussed in terms of short-term and long-term controls. This discussion will focus on the following areas: * * * * Role of the central nervous system Pregastric factors Gastrointestinal and postabsorptive factors Long-term controls Role of the Central Nervous System For many years, the hypothalamus was thought to be the key to control of food intake. This view derived from classic experiments in which food intake was studied in rats with lesions in various areas of the brain. Such studies clearly identified two regions in the hypothalamus that dramatically influence feeding behavior: * Lateral hypothalamus (hunger center): animals with lesions in this area become anorectic and lose weight. * Ventromedial hypothalamus (satiety center): animals with lesions in this area overeat and become obese. Subsequent studies showed that, although these hypothalamic centers are clearly very important in controlling hunger and satiety, they don't explain the whole story. Pregastric Factors We all know of "environmental" conditions that can dramatically affect food intake. Consider which of the following items are likely important to animals, humans or both: * Appearance of food: humans like or dislike certain meals based on visual appearance, but does your cat appreciate your buying fish-shaped food? * Taste and/or odor of food: this is extremely important in all species. * Learned preferences and aversions: Almost everyone has an aversion to one or more types of foods, and they also affect companion animals. * Psychologic factors: mental states such as fear, depression and social interactions often affect food intake. Never eat a live mole! This seagull did, the mole tried to tunnel out and they both died. Understanding these factors is of particular importance to clinicians because they can be manipulated to manage anorectic patients. Gastrointestinal and Postabsorptive Factors The degree of gastrointestinal fill is the most important signal from the digestive tract per se - a full stomach and intestine induce satiety, probably via the vagus nerve relaying that fact back to the hypothalamus. Additionally, the enteric hormone cholecystokinin is well documented to induce satiety in experimental settings. As nutrients such as glucose and amino acids are absorbed, their concentrations in blood rise, as do the concentration of several hormones (cholecystokinin as mentioned above, but also insulin and glucagon). These changes also have been linked to the sensation of hunger or satiety. Long-term Control of Food Intake Adult animals tend to maintain a relatively constant weight known as their "set weight." Much of this appears to be regulated on a time scale of weeks or longer. If an animal is starved for a long period of time, then allowed access to food, it eats a far greater amount of food than a normal animal. Conversely, if an animal is force fed for several weeks, then allowed access to free choice food, it will not eat very much. In both cases, when weight returns to "set weight," feeding behavior normalizes. An additional interesting observation is that when food is restricted, basal metabolic rate decreases, which is one reason that it is so difficult to lose weight by dieting. It is clear that long term regulation of body weight results from a complex integration of a battery of hormonal, metabolic and neural signals. In view of how tightly body weight is regulated in the face of widely varying levels of food intake and energy expenditure, it is clear that robust feedback systems are in place. Seaching for the feedback signals - "satiety factors" - has been a holy quest in this field for many years and has recently borne fruit, thanks to studies conducted years ago on mice with genetic mutations that cause obesity. The satiety factor studied most extensively to date is the hormone leptin, which has the following basic characteristics: * Leptin is synthesized and secreted almost exclusively by fat cells (adipocytes). * A major site of leptin receptors is in the hypothalamus, which is known to play an important role in control of food intake and metabolic rate. * Plasma levels of leptin rise and fall in parallel with body fat content - as body fat mass increases, so does the concentration of leptin in blood. * Injection of leptin leads to reduction in body weight by suppressing food intake and increasing metabolic rate and energy expenditure. Several other genes have been isolated that encode proteins that affect food intake, energy metabolism and body weight. Right now it is difficult to predict their future role in the pharmaceutical control of obesity, but needless to say, a number of companies are betting multimillions that one of more of these proteins will become the miracle drug for treatment of obesity. Physiology of Taste -----------------------------------------------------------------------One cannibal to another while eating a clown: "Does this taste funny to you?" The sense of taste is mediated by groups of cells called taste buds which sample oral concentrations of a large number of small molecules and report a sensation of taste to centers in the brainstem. In most animals, including humans, taste buds are most prevalent on small pegs of epithelium on the tongue called papillae. The taste buds themselves are too small to see without a microscope, but papillae are readily observed by close inspection of the tongue's surface. To make them even easier to see, put a couple of drops of blue food coloring on the tongue of a loved one, and you'll see a bunch of little light colored bumps mostly fungiform papillae - stand out on a blue background. In addition to signal transduction by taste buds, it is also clear that the sense of smell profoundly affects the sensation of taste. Think about how tastes are blunted and sometimes different when your sense of smell is disrupted due to a cold. The sense of taste is equivalent to excitation of taste receptors, and receptors for a large number of specific chemicals have been identified that contribute to the reception of taste. These include receptors for such chemicals as sodium, potassium, chloride, glutamate and adenosine. Despite this complexity, five types of tastes are commonly recognized: * * * * * Salty Sour Sweet Bitter Umami The umami taste is that of monosodium glutamate and has recently been recognized as a unique taste, as it cannot be elicited by any combination of the other four taste types. Glutamate is present in a variety of protein-rich foods, and particularly abundant in aged cheese. Perception of taste also appears to be influenced by thermal stimulation of the tongue. In some people, warming the front of the tongue produces a clear sweet sensation, while cooling leads to a salty or sour sensation. It should be noted that these tastes are based on human sensations and some comparative physiologists caution that each animal probably lives in its own "taste world". For animals, it may be more appropriate to discuss tastes as being pleasant, unpleasant or indifferent. None of these tastes are elicited by a single chemical. Also, there are thresholds for detection of taste that differ among chemicals that taste the same. For example, sucrose, 1-propyl-2 amino-4-nitrobenzene and lactose all taste sweet to humans, but the sweet taste is elicited by these chemicals at concentrations of roughly 10 mM, 2 uM and 30 mM respectively - a range of potency of roughly 15,000-fold. Substances sensed as bitter typically have very low thresholds. Examples of some human thresholds Taste Substance Threshold for tasting Salty NaCl 0.01 M Sour HCl 0.0009 M Sweet Sucrose 0.01 M Bitter Quinine 0.000008 M Umami Glutamate 0.0007 M There are also differences among species in location of taste buds - in dogs, they are located predominantly on the anterior part of the tongue, while in cattle most are on the posterior tongue. Taste buds are composed of groups of about 40 columnar epithelial cells bundled together along their long axes. Taste cells within a bud are arranged such that their tips form a small taste pore, and through this pore extend microvilli from the taste cells. The microvilli of the taste cells bear taste receptors and it appears that most taste buds contain cells that bear receptors for 2 or 3 of the basic tastes. Interwoven among the taste cells in a taste bud is a network of dendrites of sensory nerves called "taste nerves." When taste cells are stimulated by binding of chemicals to their receptors, they depolarize and this depolarization is transmitted to the taste nerve fibers resulting in an action potential that is ultimately transmitted to the brain. One interesting aspect of this nerve transmission is that it rapidly adapts - after the initial stimulus, a strong discharge is seen in the taste nerve fibers but within a few seconds, that response diminishes to a steady-state level of much lower amplitude. Once taste signals are transmitted to the brain, several efferent neural pathways are activated that are important to digestive function. For example, tasting food is followed rapidly by increased salivation and by low level secretory activity in the stomach. Considerable attention has been devoted to understanding the benefits to survival and wellbeing that accrue from having a sense a taste. Some have speculated that an ability to taste bitterness may protect animals from ingesting certain natural poisons. There is no doubt that animals, including humans, develop taste preferences. That is, they will choose certain types of food in preference to others. Interestingly, taste preference often changes in conjunction with body needs. Similarly animals often develop food aversions, particularly if they become ill soon after eating a certain food, even though that food was not the cause of the illness - surely you have experienced this yourself. Food preferences and aversions involve the sense of taste, but these phenomena are almost certainly mediated through the central nervous system rather that directly through taste cells. Salivary Glands and Saliva -----------------------------------------------------------------------Saliva is produced in and secreted from salivary glands. The basic secretory units of salivary glands are clusters of cells called an acini. These cells secrete a fluid that contains water, electrolytes, mucus and enzymes, all of which flow out of the acinus into collecting ducts. Within the ducts, the composition of the secretion is altered. Much of the sodium is actively reabsorbed, potassium is secreted, and large quantities of bicarbonate ion are secreted. Bicarbonate secretion is of tremendous importance to ruminants because it, along with phosphate, provides a critical buffer that neutralizes the massive quantities of acid produced in the forestomachs. Small collecting ducts within salivary glands lead into larger ducts, eventually forming a single large duct that empties into the oral cavity. Most animals have three major pairs of salivary glands that differ in the type of secretion they produce: * parotid glands produce a serous, watery secretion * * submaxillary (mandibular) glands produce a mixed serous and mucous secretion sublingual glands secrete a saliva that is predominantly mucous in character The basis for different glands secreting saliva of differing composition can be seen by examining salivary glands histologically. Two basic types of acinar epithelial cells exist: * serous cells, which secrete a watery fluid, essentially devoid of mucus * mucous cells, which produce a very mucus-rich secretion In the histologic sections of canine salivary gland shown above, the cells stained pink are serous cells, while the white, foamy cells are mucus-secreting cells. Acini in the parotid glands are almost exclusively of the serous type, while those in the sublingual glands are predominantly mucus cells. In the submaxillary glands, it is common to observe acini composed of both serous and mucus epithelial cells. Secretion of saliva is under control of the autonomic nervous system, which controls both the volume and type of saliva secreted. This is actually fairly interesting: a dog fed dry dog food produces saliva that is predominantly serous, while dogs on a meat diet secrete saliva with much more mucus. Parasympathetic stimulation from the brain, as was well demonstated by Ivan Pavlov, results in greatly enhanced secretion, as well as increased blood flow to the salivary glands. Potent simuli for increased salivation include the presence of food or irritating substances in the mouth, and thoughts of or the smell of food. Knowing that salivation is controlled by the brain will also help explain why many psychic stimuli also induce excessive salivation for example, why some dogs salivate all over the house when it's thundering What then are the important functions of saliva ? Actually, saliva serves many roles, some of which are important to all species, and others to only a few: * Lubrication and binding: the mucus in saliva is extremely effective in binding masticated food into a slippery bolus that (usually) slides easily through the esophagus without inflicting damage to the mucosa. Saliva also coats the oral cavity and esophagus, and food basically never directly touches the epithelial cells of those tissues. * Solubilizes dry food: in order to be tasted, the molecules in food must be solubilized. * Oral hygiene: The oral cavity is almost constantly flushed with saliva, which floats away food debris and keeps the mouth relatively clean. Flow of saliva diminishes considerably during sleep, allow populations of bacteria to build up in the mouth -- the result is dragon breath in the morning. Saliva also contains lysozyme, an enzyme that lyses many bacteria and prevents overgrowth of oral microbial populations. * Initiates starch digestion: in most species, the serous acinar cells secrete an alphaamylase which can begin to digest dietary starch into maltose. Amylase is not present or present in very small quantities in the saliva of carnivores or cattle. * Provides alkaline buffering and fluid: this is of great importance in ruminants, which have non-secretory forestomachs. * Evaporative cooling: clearly of importance in dogs, which have very poorly developed sweat glands - look at a dog panting after a long run and this function will be clear. Diseases of the salivary glands and ducts are not uncommon in animals and man, and excessive salivation is a symptom of almost any lesion in the oral cavity. The dripping of saliva seen in rabid animals is not actually a result of excessive salivation, but due to pharyngeal paralysis, which prevents saliva from being swallowed. Prehension, Mastication, Swallowing -----------------------------------------------------------------------Prehension is the process of siezing or grasping or otherwise getting food into the mouth. Different species use different techniques to prehend food - for example, horses and goats rely considerably on their lips, whereas cattle, dogs and cats don't use their lips to any extent, but rather, gather many foods with their tongues. Studying comparative prehension can be entertaining, but is of minimal value for understanding digestion. As with prehension, there are considerable differences among species in techniques used for drinking, that basically boil down to being either a "sucker" or a "lapper". Drinking is usually an efficient process, although beards and moustaches can sometimes interfere. Mastication, or chewing, is the first step in the breakdown of complex foodstuffs and serves several functions, including: * breaking large pieces into small pieces, resulting in a massive increase in surface area, which is where digestive enzymes work * softening of food and transformation into a size conducive to swallowing * lubrication of food by impregnating it with saliva Chewing is, to a large extent, a reflex. To study this phenomenon, watch a cow ruminating or look around and watch someone chewing gum. The presence of food (or gum) in the mouth causes a reflex inhibition of the muscles of the lower jaw. Those muscles relax and the lower jaw drops, causing a stretch reflex which causes muscle contraction and closure of the mouth. During mastication, the tongue and, to a lesser extent, the lips and cheeks acts to keep food between the grinding surfaces of the teeth. This can be demonstrated by trying to chew your next meal while holding your tongue still. Incidentally, chewing is hard work and expends a lot of energy. Deficits in the ability to effectively masticate are a very common cause of digestive disease in animals. Many of these problems are associated with poor teeth, and most are easily diagnosed by simple inspection. A particularly common problem in horses is the occurence of "points" on the molar teeth. The final step in pregastric digestion is swallowing, also known as deglutition. This is really a very complex process that can be thought of as occurring in three steps: * First, a bolus of food is pressed backward into the pharynx by the tongue. This is the only step that is voluntary - the remaining steps occur by reflex. * Once the bolus reaches the pharynx several actions are initiated, which basically involve shunting the bolus into the esophagus while at the same time closing alternative routes of escape. The lumen of the larynx is squeezed shut and the epiglottis swings backward to cover the larynx. The larynx is also pulled forward and down making the opening to the esophagus larger. * Finally, the tongue presses backward and a peristaltic contraction in the pharynx propels the bolus into the esophagus, where the actual act of swallowing takes place. The Esophagus -----------------------------------------------------------------------Anatomically and functionally, the esophagus is the least complex section of the digestive tube. Its role in digestion is simple: to convey boluses of food from the pharynx to the stomach. Like other parts of the digestive tube, it has four tunics, but important differences exist in the composition of these tunics in comparison to more distal sections of the tube. First, instead of the muscular tunic being entirely smooth muscle, as it is in the stomach and intestines, the wall of the esophagus contains a variable amount of striated muscle. In dogs, cattle and sheep, its entire length is striated muscle, whereas in cats, horses and humans, the proximal esophagus has striated muscle and the distal esophagus smooth muscle. Second, instead of the esophagus being free as it courses through the thoracic cavity, it is embedded in the connective tissue; thus, its outer tunic is referred to as adventitia instead of serosa. In its role as the first conduit in the digestive tube, the esophagus is routinely exposed to rough and abrasive foodstuffs, like fragments of bone, fibrous plant leaves and Doritos. Its surface should therefore be resistant to trauma, and indeed, the esophagus is lined with stratified squamous epithelium, as seen below in an image from a cat's esophagus: Absorption in the esophagus is virtually nil, but the mucosa does contain a few mucous glands that, as you might expect, secrete mucus to aid in lubrication. The body of the esophagus is bounded by physiologic sphincters known as the upper and lower esophageal sphincters. The upper sphincter is composed largely of a muscle that is closely associated with the larynx. When relaxed, as it is during swallowing, this muscle pulls the larynx forward and aids in routing food into the esophagus instead of the larynx. The lower sphincter is simply the muscle that surrounds the esophagus just as it enters the stomach. Both the upper and lower sphincter are closed except during swallowing, which prevents constant entry of air from the oral cavity or reflux of stomach contents. During swallowing, boluses of food are propelled through the esophagus by strong peristaltic contractions. In dogs and humans, it takes 4-5 seconds for the bolus to traverse the esophagus. If the bolus is not delivered in "one pass", secondary waves of peristalsis are initiated at the point of distention, which almost always result in delivery of the bolus to the stomach. Congenital and acquired disorders in esophageal motility that interfere with this usually reliable delivery of food are rather common in both animals and man. Genetics of Food Intake, Body Weight and Obesity -----------------------------------------------------------------------It has been clear for several decades that maintenance of body weight is under genetic control, largely due to identification of mutations in mice that result in obesity. Recently, amid great excitement, several such genes have been cloned from mice and humans, and it's very likely that additional genetic determinants will soon be identified. The incentive to understand genetic control over body weight can largely be attributed to two factors: * Obesity is a monumental problem in the developed world and the allure of finding a drug to cure this problem is strong. * There is a strong association between development of obesity in adulthood and development of other important diseases, including diabetes, hypertension and heart disease. Initial Identification of "Obesity Genes" To understand the physiology behind the "obesity genes" currently under investigation, it is valuable to first look back at some experiments conducted in the 1960's using parabiotic mice. The technique of parabiosis, which is rarely used today, involves making an incision along the lateral aspect of two animals, then suturing them together to form a parabiotic pair. The key utility of this technique is that it unites the vascular systems of the two animals, allowing exchange of blood-borne molecules. Many years ago, geneticists identified in mice two recessive mutations which, if homozygous, led the mice to become grossly obese. The two genes were termed ob and db. Parabiotic pairs constructed between ob/ob, db/db and normal mice led to the following observations: * Pairing an obese ob/ob mouse with a normal mouse: the ob/ob mouse lost weight * Pairing an obese db/db mouse with a normal mouse: the normal mouse stopped eating and lost weight * Pairing an obese ob/ob mouse with an obese db/db mouse: the ob/ob mouse stopped eating and lost weight, whereas the db/db mouse was unaffected. * An additional experiment showed that when one of a pair of normal parabiotic mice was overfed, its "twin" lost weight. These observations were consistent with the idea that a satiety hormone, presumably the ob gene product, is produced which binds to receptors, presumably the db gene product, in the hypothalamus and suppresses hunger. Considerable support was recently obtained for this model by the cloning of the ob and db genes from several species. The ob gene encodes the hormone leptin and the db gene the leptin receptor. Leptin is secreted by fat cells and has dual activity of decreasing food intake and increasing metabolic rate, which makes the old "lipostatic theory" for control of food intake very appealing. Genes Involved in Maintaining Body Weight It is clear that leptin and its receptor are only two of what may turn out to be a large number of genes that are important genetic determinants in the control of body weight and pathogenesis of obesity. Some of the other genes and gene products identified so far that are involved in control of food intake and body weight include: * Neuropeptide Y is synthesized in many areas of the brain and is a potent stimulator of feeding behavior. Leptin appears to suppress feeding in part by inhibiting expression of neuropeptide Y. * Melanocortins effect certain hypothalamic neurons and inhibit of feeding behavior. Targeted disruptions of the melanocortin-4 receptor in mice are associated with development of obesity. * Carboxypeptidase E (fat gene) is the enzyme necessary for proteolytic processing of proinsulin and perhaps other hormones such as neuropeptide Y. Mice with mutations in this gene gradually become obese as they age, and develop hyperglycemia that can be suppressed by treatment with insulin. * Mitochondrial uncoupling proteins were first discovered in brown fat, and subsequently identified in white fat and muscle cells. They allow mitochondria within those cells to uncouple oxidative phosphorylation, which "short circuits" the proton gradient across the inner membrane, leading to diminished production of ATP, but generating heat (nonshivering thermiogenesis). Some research suggests that they may play an important role in energy expenditure and thus body weight in man and other non-hybernating animals. * Beta-adrenergic receptors are present on brown fat and perhaps white fat. Binding of norepinephrine to this receptor on fat cells leads to increased transcription of the mitochondrial uncoupling protein, allowing increased heat production via hydrolysis of fatty acids. It was recently reported that certain mutations in this gene predisposed people to become obese and develop diabetes before middle age. * Tubby protein, along with tubby-related proteins, are presumed transcription factors. Tubby protein is highly expressed the paraventricular nucleus of the hypothalamus and other regions of the brain. Mice with naturally-occurring or engineered mutations in the tubby gene show adult onset of obesity, but the mechanisms involved are not known. ------------------------------------------------------------------------References and Reviews Taste Preferences -----------------------------------------------------------------------Taste preference in the face of specific deficiencies is actually quite interesting. Some examples include: * Removal of the adrenal glands without replacement of mineralocorticoids leads rapidly to death due to massive loss of sodium from the body. Adrenalectomized animals show a clear preference for salty water over pure water, and if provided with salt water, can actually survive. * If the parathyroid glands are removed, animals loose calcium and cannot maintain blood calcium levels appropriately due to deficiency in parathyroid hormone. Following parathyroidectomy, animals choose drinking water that contains calcium chloride over pure water or water containing equivalent concentrations of sodium chloride. * Injection of excessive doses of insulin results in hypoglycemia (low blood sugar). Following such treatment, animals will preferentially pick out and consume the sweetest among a group of foods. Dental Anatomy -----------------------------------------------------------------------Teeth are very important to an animal as they are used for eating, grooming and defense. Consequently, dental problems, if not treated, often lead to more generalized illness. Mammals have teeth of different sizes and shapes, a condition known as heterodonty, allowing different teeth to be specialized for different tasks. These specialized teeth include: * * * * Incisors (I) Canine teeth (C) Premolars (P) Molars (M) Mammals also have two sets of teeth: a deciduous set (milk teeth, baby teeth) and a permanent set. Dental Formulae Dental formulae are used to indicate the number of each type of tooth for a given species. Because the jaw is bilaterally symetrical, only one half of the jaw is described. The incisors are indicated first, followed by the canine, promolars and molars. The maxillary arcade or upper jaw is listed over the mandibular arcade or lower jaw. For instance, a dog has 3 incisors, 1 canine, 4 premolars and 2 molars on one side of the upper jaw and 3 incisors, 1 canine, 4 premolars and 3 molars on one side of the lower jaw, so the dental formula would be: upper : I C P M : 3142 lower I C P M 3143 Individual teeth can also be denoted: The first lower incisor would be I1 and the second upper molar would be M2. Maxillary Arcade of the Dog Anatomy of Teeth There is considerable variation in dental anatomy among animals. In mammals, there are two distictive types of teeth that differ in pattern of growth and morphology: Brachydont or low-crowned teeth are what is seen in man, carnivores such as dogs and cats, and pigs. This type of tooth consists of a crown above the gingiva, a constricted neck at the gum line, and a root embedded in the jawbone. The crown is encased in enamel and the root in cementum. Enamel is the hardest substance in the body being densely packed with hydroxyapatite (mineral) crystal and heavily mineralized with calcium salts. Cementum is calcified connective tissue. Dentin, a bonelike material, is under the enamel and makes up most of the tooth. The pulp cavity includes blood vessels, lymphatics and nerves. Hypsodont or high-crowned teeth are continue to erupt throughout life. Examples of this type of teeth include all of the permanent teeth of horses and cheek teeth of ruminants. Hypsodont teeth are usually described as having a body, much of which is below the gum line, and root, which is embedded in the alveolus of the jaw bone. Enamel covers the entire body of the tooth, but not the root. In both high-crowned and low-crowned teeth, the tooth is attached to a "socket" in the jaw bone called an alveolus. The attachment is through a fibrous capsule called the gomphosis. Remembering the term gomphosis is required only of dental students. An additional type of nomenclature is used to describe the different surfaces of teeth, as depicted in the image below. The occlusal surface is the chewing surface. Dental Anatomy of specific species * Humans * * * * * * * * Cats Dogs Horses Llamas Rabbits Rodents Ruminants Swine Chewing - All In a Day's Work -----------------------------------------------------------------------It turns out that chewing expends quite a lot of energy. Cattle spend a large part of their lives chewing. When they are consuming high quality feed, roughly 10% of the energy content of the feed is expended in the process of chewing it. Put cattle on low quality feed, such as wheat straw, and that figure jumps to about 25% of energy content (Susenbeth et al. 1998). Similar values have been measured in horses. What about chewing gum? In a recent study by Levine and colleagues (1999), energy expenditure was measured in 7 non-obese volunteers during chair rest, and while sitting in the same chair chewing calorie-free gum at a rate of 100 MPM (mastications per minute). The basal metabolic rate at rest was 58 + 11 kcal per minute. During a 12 minute episode of chewing, metabolic rate rose to 70 + 14 kcal per minute, an increase over resting of approximately 20%. As a comparision, walking a mile in an hour basically doubles energy expenditure over resting values. So, can chewing gum be used as a weight-control strategy? It's not a completely silly idea the authors cited above calculated that by chewing calorie-free gum during waking hours and not changing any other aspect of energy balance, a person should lose about 5 kg of fat per year. Can you envision a gum room next to the weights room at your local health club? "Well, that's enough for me, I'm off to the showers." -----------------------------------------------------------------------Foodstuffs entering the stomach have been, to at least some extent, crushed and reduced in size by mastication, and impregnanted with saliva. The stomach provides four basic functions that assist in the early stages of digestion and prepare the ingesta for further processing in the small intestine: 1. It serves as a short-term storage reservoir, allowing a rather large meal to be consumed quickly and dealt with over an extended period of time. 2. It is in the stomach that substantial chemical and enzymatic digestion is initiated, particularly of proteins. 3. Vigorous contractions of gastric smooth muscle mix and grind foodstuffs with gastric secretions, resulting in liquefaction of food, a prerequisite for delivery of the ingesta to the small intestine. 4. As food is liquefied in the stomach, it is slowly released into the small intestine for further processing. Core gastric physiology is presented as the following topics: * * * * * Gross and microscopic anatomy of the stomach Gastric motility - filling and emptying Gastric secretions Absorption in the stomach One meal in the life of the stomach Advanced and supplemental topics related to gastric physiology: * * * * * * * * Histology of the Stomach Secretory Processes The Parietal Cell and the Mechanism of Acid Secretion Drug Therapy for Suppressing Secretion of Gastric Acid Pepsin and Pepsinogens Rennin and the Coagulation of Milk Intrinsic Factor Enterochromaffin-Like (ECL) Cells * * * * Gastric Motility Control of Gastric Emptying The Migrating Motor Complex Physiology of Vomiting * The Gastrointestinal Barrier Gross and Microscopic Anatomy of the Stomach -----------------------------------------------------------------------The stomach is an expanded section of the digestive tube between the esophagus and small intestine. It's characteristic shape is shown, along with terms used to describe the major regions of the stomach. The right side of the stomach shown above is called the greater curvature and that on the left the lesser curvature. The most distal and narrow section of the stomach is termed the pylorus - as food is liquefied in the stomach it passes through the pyloric canal into the small intestine. The wall of the stomach is structurally similar to other parts of the digestive tube, with the exception that the stomach has an extra, oblique layer of smooth muscle inside the circular layer, which aids in performance of complex grinding motions. In the empty state, the stomach is contracted and its mucosa and submucosa are thrown up into distinct folds called rugae; when distended with food, the rugae are "ironed out" and flat. The image to the right shows rugae on the surface of a dog's stomach. Within the stomach there is an abrupt transition from stratified squamous epithelium extending from the esophagus to a columnar epithelium dedicated to secretion. In most species, this transition is very close to the esophageal orifice, but in some, particular horses and rodents, stratified squamous cells line much of the fundus and part of the body. The image to the right is of the mucosal surface of an equine stomach showing esophageal epithelium (top) and glandular epithelium (bottom). The creatures attached to the surface are bots, larval forms of Gasterophilus. If the lining of the stomach is examined with a hand lens, one can see that it is covered with numerous small holes. These are the openings of gastric pits which extend into the mucosa as straight and branched tubules, forming gastric glands. Four major types of secretory epithelial cells cover the surface of the stomach and extend down into gastric pits and glands: * Mucous cells: secrete an alkaline mucus that protects the epithelium against shear stress and acid * Parietal cells: secrete hydrochloric acid! * Chief cells: secrete pepsin, a proteolytic enzyme * G cells: secrete the hormone gastrin There are differences in the distribution of these cell types among regions of the stomach - for example, parietal cells are abundant in the glands of the body, but virtually absent in pyloric glands. The micrograph to the right shows a gastric pit invaginating into the mucosa (fundic region of a raccoon stomach). Notice that all the surface cells and the cells in the neck of the pit are foamy in appearance - these are the mucous cells. The other cell types are farther down in the pit and, in this image, difficult to distinguish. Gastric Motility: Filling and Emptying -----------------------------------------------------------------------Contractions of gastric smooth muscle serves two basic functions: * ingested food is crushed, ground and mixed, liquefying it to form what is called chyme. * chyme is forced through the pyloric canal into the small intestine, a process called gastric emptying. The stomach can be divided into two regions on the basis of motility pattern: an accordianlike reservoir that applies constant pressure on the lumen and a highly contractile grinder. The upper stomach, composed of the fundus and upper body, shows low frequency, sustained contractions that are responsible for generating a basal pressure within the stomach. Importantly, these tonic contractions also generate a pressure gradient from the stomach to small intestine and are thus responsible for gastric emptrying. Interestingly, swallowing of food and consequent gastric distention inhibits contraction of this region of the stomach, allowing it to balloon out and form a large reservoir without a significant increase in pressure. The lower stomach, composed of the lower body and antrum, develops strong peristaltic waves of contraction that increase in amplitude as they propagate toward the pylorus. These powerful contractions constitute a very effective gastric grinder; they occur about 3 times per minute in people and 5 to 6 times per minute in dogs. Gastric distention strongly stimulates this type of contraction, accelerating liquefaction and hence, gastric emptying. The pylorus is functionally part of this region of the stomach - when the peristaltic contraction reaches the pylorus, its lumen is effectively obliterated - chyme is thus delivered to the small intestine in spurts. Gastric motility is controlled by a very complex set of neural and hormonal signals. Nervous control originates from the enteric nervous system as well as parasympathetic (predominantly vagus nerve) and sympathetic systems. A large battery of hormones have been shown to influence gastric motility - for example, both gastrin and cholecystokinin act to relax the proximal stomach and enhance contractions in the distal stomach. The bottom line is that the patterns of gastric motility likely are a result from smooth muscle cells integrating a large number of inhibitory and stimulatory signals. Liquids readily pass through the pylorus in spurts, but solids must be reduced to a diameter of less than 1-2 mm before passing the pyloric gatekeeper. Larger solids are propelled by peristalsis toward the pylorus, but then refluxed backwards when they fail to pass through the pylorus - this continues until they are reduced in size sufficiently to flow thought the pylorus. At this point, you may be asking "What happens to solids that are indigestible - for example, a rock or a penny? Will it remain forever in the stomach?" If the indigestible solids are large enough, they indeed cannot pass into the small intestine, and will either remain in the stomach for long periods, induce a gastric obstruction or, as every cat owner knows, be evacuated by vomition. However, many of the indigestible solids that fail to pass through the pylorus shortly after a meal do pass into the small intestine during periods between meals. This is due to a different pattern of motor activity called the migrating motor complex, a pattern of smooth muscle contractions that originates in the stomach, propagates through the intestines and serves a housekeeping function to periodically sweep out the gastroi Gastric Secretions -----------------------------------------------------------------------The stomach is famous for its secretion of acid, but acid is only one of four major secretory products of the gastric epithelium, all of which are important either to the digestive process or to control of gastric function: Mucus, Hydrochloric acid, Pepsinogen, an inactive zymogen, Hormones(gastrin, rennin) * Mucus: The most abundant epithelial cells are mucous cells, which cover the entire lumenal surface and extend down into the glands as "mucous neck cells". These cells secrete a bicarbonate-rich mucus that coats and lubricates the gastric surface, and serves an important role in protecting the epithelium from acid and other chemical insults. * Acid: Hydrochloric acid is secreted from parietal cells into the lumen where it establishes an extremely acidic environment. This acid is important for activation of pepsinogen and inactivation of ingested microorganisms such as bacteria. * Proteases: Pepsinogen, an inactive zymogen, is secreted into gastric juice from both mucous cells and chief cells. Once secreted, pepsinogen is activated by stomach acid into the active protease pepsin, which is largely responsible for the stomach's ability to initiate digestion of proteins. In young animals, chief cells also secrete chymosin (rennin), a protease that coagulates milk protein allowing it to be retained more than briefly in the stomach. * Hormones: The principle hormone secreted from the gastric epithelium is gastrin, a peptide that is important in control of acid secretion and gastric motility. A number of other enzymes are secreted by gastric epithelial cells, including a lipase and gelatinase. One secretory product of considerable importance in man is intrinsic factor, a glycoprotein secreted by parietal cells that is necessary for intestinal absorption of vitamin B12. Absorption in the Stomach -----------------------------------------------------------------------The stomach absorbs very few substances, although small amounts of certain lipid-soluble compounds can be taken up, including aspirin, other non-steroidal anti-infammatory drugs, and ethanol. Notably, these substances are also well-recognized causes of gastric irritation and their use (especially overuse) is commonly associated with development of gastritis and gastric ulcers. One Meal in the Life of the Stomach -----------------------------------------------------------------------The stomach functions dynamically, in parallel with meals. Consider the stomach's most notable activity - secretion of acid. Acid is secreted in large quantities when the stomach is distended with food, which is useful because it facilitates the initial breakdown of proteins. However, once the meal has been liquefied and the stomach has emptied, acid secretion trickles to a stop and remains shut off during the interdigestive period. This shut-off in acid secretion is a good thing - otherwise excessive acid would damage the mucosa of the stomach and small intestine, as happens in certain disease states. Gastric function is often classified into three phases in which secretory and motor activities are tightly coupled. Try identifying these phases in yourself or your loved ones around meal time: Cephalic phase ("wake up call"): Seeing, smelling and anticipating food in perceived in the brain and the brain informs the stomach that it should prepare for receipt of a meal. This communication is composed of parasympathetic stimuli transmitted thought the vagus nerve to the enteric nervous system, resulting in release of acetylcholine in the vicinity of G cells and parietal cells. Binding of acetylcholine to its receptor on G cells induces secretion of the hormone gastrin, which, in concert with acetylcholine and histamine, stimulates parietal cells to secrete small amounts of acid. Additionally, a low level of gastric motility is induced. In essense, the gastric motor is turned on and begins to idle. Gastric phase ("full steam ahead"): When a meal enters the stomach several additional factors come into play, foremost among them distension and mucosal irritation. Distension excites stretch receptors and irritation activates chemoreceptors in the mucosa. These events are sensed by enteric neurons, which secrete additional acetylcholine, further stimulating both G cells and parietal cells; gastrin from the G cells feeds back to the parietal cells, stimulating it even further. Additionally, activation of the enteric nervous system and release of gastrin cause vigorous smooth muscle contractions. The net result is that secretory and motor functions of the stomach are fully turned on lots of acid and pepsinogen are secreted, pepsinogen is converted into pepsin and vigorous grinding and mixing contractions take place. However, there is a mechanism in place in the stomach to prevent excessive acid secretion - if lumenal pH drops low enough (less than about 2), motility and secretion are temporarily suspended. Intestinal phase ("step on the brakes"): As food is liquefied in the stomach, it is emptied into the small intestine. Its seems to be important for the small intestine to be able to slow down gastric emptying, probably to allow it time to neutralize the acid and efficiently absorb incoming nutrients. Hence, this phase of gastric function is dominated by the small intestine sending inhibitory signals to the stomach to slow secretion and motility. Two types of signals are used: nervous and endocrine. Distension of the small intestine, as well as chemical and osmotic irritation of the mucosa is transduced into gastric-inhibitory impulses in the enteric nervous system - this nervous pathway is called the enterogastric reflex. Secondly, enteric hormones such as cholecystokinin and secretin are released from cells in the small intestine and contribute to suppression of gastric activity. Collectively, enteric hormones and the enterogastric reflex put a strong brake on gastric secretion and motility. As the ingesta in the small intestine is processed, these stimuli diminish, the damper on the stomach is released, and its secretory and motor activities resume. To summarize, the brain alerts the stomach that it should expect arrival of a meal and the stomach comes out of its interdigestive quiescence and begins low level motor and secretory activity (cephalic phase). After a meal is consumed, the gastric motor and secretory activity is fully turned on (gastric phase). If the meal is at all substantial, the gastric phase is periodically suppressed by signals from the small intestine and, if gastric pH falls to very low levels, from the stomach itself. Eventually, the meal is fully liquefied and emptied, and the stomach falls back into a state of very low motor and secretory activity, where it remains until the next cephalic phase. Drug Therapy for Suppressing Secretion of Gastric Acid -----------------------------------------------------------------------Understanding the mechanisms involved in secretion of acid from the parietal cell led to development of several drugs capable of inhibiting acid secretion. The most effective inhibitors fall into two classes. H2 Receptor Antagonists Despite some unresolved questions, it is clear that histamine is one of the primary regulators of acid secretion. The parietal cell receptor for histamine is of the H2 type, and antihistamines that engage H1 receptors have no effect on acid secretion. Evidence of histamine's role in acid secretion is strongly supported by finding that H2 receptor antagonists are quite effective in inhibiting acid secretion. Four primary H2 antagonists have been developed and found clinical utility: * * * * cimetidine ranitidine famotidine nizatidine These drugs, particularly cimetidine, are among the most widely prescribed drugs in man. They are also useful for management of certain gastric diseases in dogs and horses. Proton Pump Inhibitors Acid secretion is absolutely dependent on function of the H+, K+ ATPase or proton pump located in the cannilicular membrane of the parietal cell. Several drugs have been developed that non-competively bind and inactivate the ATPase, resulting in strong inhibition of acid secretion. Omeprazole is an acid-activated prodrug that binds covalently to two cysteines on the ATPase, resulting in its irreversible inactivation. Two other inhibitors, lansoprazole and pantoprazole have similar modes of action. ------------------------------------------------------------------------References and Reviews Pepsinogens and Pepsins -----------------------------------------------------------------------Pepsins are the principal proteases in gastric secretions of adult mammals. They are members of the family of aspartic proteases, and closely related to chymosin, another gastric protease expressed particularly in young animals. These enzymes are synthesized and secreted predominantly by chief cells in the gastric mucosa. At least 8 isozymes of pepsinogen have been identified in gastric epithelial cells, and these have been categorized into two immunologically-separable types (pepsins A and C). The mature, active enzymes are roughly 325 amino acids with a mass of approximately 35 kDa. Pepsins are synthesized as inactive pre-proenzymes, consisting of a signal peptide, activation peptide and active enzyme. The signal peptide is cleaved as the protein is inserted into endoplasmic reticulum and the resulting proenzyme - pepsinogen - is transported to the Golgi and condensed into secretory granules. Pepsinogens are secreted in a form such that the activation peptide assumes a compact structure that occludes the active site. On exposure to an acidic (pH < 4) environment such as occurs in the lumen of the stomach, the activation peptide unfolds, allowing the active site to clip it off, yielding mature, catalytically active pepsin. Structurally, the active site is located in a deep cleft within the molecule. Optimal activity of pepsins is at pH of 1.8 to 3.5, depending on the isoform. They are reversibly inactivated at about pH 5 and irreversibly inactivated at pH 7 to 8. Wireframe model of porcine pepsin (If your browser has a pdb viewer like Rasmol, click here to view [230 kb]) In general, secretion of pepsinogens is coupled to secretion of acid from the parietal cell. In vitro studies have demonstrated that secretion is effectively stimulated by agents that stimulate either of two conditions: * Elevated intracellular levels of cyclic AMP: examples include secretin, vasoactive intestinal peptide and epinephrine. * Elevated intracellular calcium: the principal mediators investigated include acetylcholine and peptides of the gastrin/cholecystokinin family Receptors for many of the hormones listed above have been demonstrated on chief cells and pepsinogen secretion has been stimulated or blocked by exposure to these agents or their antagonists, respectively. At the present time it seems safe to say that the principal physiologic secretagogue(s) regulating pepsinogen secretion has not been clearly deliniated. Control of Gastric Emptying -----------------------------------------------------------------------The rate of gastric emptying is strongly influenced by both volume and composition of gastric contents, which makes considerable sense. Consider three examples of something you might ingest and which rate of gastric emptying would be most appropriate: * A large glass of water: The stomach becomes distended, but there are no solids to grind and liquefy, and after the water reaches the small intestine, no further processing is required before absorption - the rate of gastric emptying should be very fast. * A Double Whopper with fries (or a mouse if you're a cat): The stomach is distended and its contents must be liquefied; you would also want the meal to be retained in the stomach long enough for pepsin and acid to get a good shot at digesting the protein. Additionally, the resulting chyme should be allowed to empty in the small intestine slowly so as to not overload that organ, particularly with regard to digestion of fat - the rate of gastric emptying should be slow. * A single Chicken McNugget (or a grasshopper if you're a cat): The stomach will not be distended after this kind of a "meal" and in the absense of distension, there is relatively little stimulus for gastric motility - the rate of gastric emptying should be slow. For liquids, the principal determinant of rate of gastric emptying is volume and, secondarily, composition. If the liquid is low in nutrients (e.g. Evian bottled water), there is an exponential relationship between volume and rate of emptying - large volumes empty at an exponentially faster rate than small volumes. However, if the fluid is hypertonic or acidic or rich in nutrients such as fat or certain amino acids, the rate of gastric emptying will be considerably slower and non-exponential. Indeed, the rate of gastric emptying of any meal can be predicted rather accurately by knowing its nutrient density. Nutrient density is sensed predominantly in the small intestine by osmoreceptors and chemoreceptors, and relayed to the stomach as inhibitory neural and hormonal messages that delay emptying by altering the patterns of gastric motility. The presence of fat in the small intestine is the most potent inhibitor of gastric emptying, resulting in relaxation of the proximal stomach and diminished contractions of the distal, "gastric grinder" - when the fat has been absorbed, the inhibitory stimulus is removed and productive gastric motility resumes. Understanding the basic principles of gastric emptying facilitates management of gastric motility disorders, which are relatively common in both man and animals. -----------------------------------------------------------------------Physiology of Vomiting -----------------------------------------------------------------------At least after death you're not nauseous. Woody Allen in Sleeper Vomiting is the forceful expulsion of contents of the stomach and often, the proximal small intestine. It is a manifestation of a large number of conditions, many of which are not primary disorders of the gastrointestinal tract. Regardless of cause, vomiting can have serious consequences, including acid-base derrangments, volume and electrolyte depletion, malnutrition and aspiration pneumonia. The Act of Vomiting Vomiting is usually experienced as the finale in a series of three events, which everyone reading this has experienced: * Nausea is an unpleasant and difficult to describe psychic experience in humans and probably animals. Physiologically, nausea is typically associated with decreased gastric motility and increased tone in the small intestine. Additionally, there is often reverse peristalsis in the proximal small intestine. * Retching ("dry heaves") refers to spasmodic respiratory movements conducted with a closed glottis. While this is occurring, the antrum of the stomach contracts and the fundus and cardia relax. Studies with cats have shown that during retching there is repeated herniation of the abdominal esophagus and cardia into the thoracic cavity due to the negative pressure engendered by inspiratory efforts with a closed glottis. * Emesis or vomition is when gastric and often small intestinal contents are propelled up to and out of the mouth. It results from a highly coordinated series of events that could be described as the following series of steps (don't practice these in public): * A deep breath is taken, the glottis is closed and the larynx is raised to open the upper esophageal sphincter. Also, the soft palate is elevated to close off the posterior nares. * The diaphragm is contracted sharply downward to create negative pressure in the thorax, which facilitates opening of the esophagus and distal esophageal sphincter. * Simultaneously with downward movement of the diaphragm, the muscles of the abdominal walls are vigorously contracted, squeezing the stomach and thus elevating intragastric pressure. With the pylorus closed and the esophagus relatively open, the route of exit is clear. The series of events described seems to be typical for humans and many animals, but is not inevitable. Vomition occasionally occurs abruptly and in the absense of premonitory signs this situation is often referred to as projectile vomiting. A common cause of projectile vomiting is gastric outlet obstruction, often a result of the ingestion of foreign bodies. An activity related to but clearly distinct from vomiting is regurgitation, which is the passive expulsion of ingested material out of the mouth - this often occurs even before the ingesta has reached the stomach and is usually a result of esophageal disease. Regurgitation also is a normal component of digestion in ruminants. There is also considerable variability among species in the propensity for vomition. Rats reportedly do not vomit. Cattle and horses vomit rarely - this is usually an ominous sign and most frequently a result of acute gastric distension. Carnivores such as dogs and cats vomit frequently, often in response to such trivial stimuli as finding themselves on a clean carpet. Humans fall between these extremes, and interestingly, rare individuals have been identified that seem to be incapable of vomiting due to congenital abnormalities in the vomition centers of the brainstem. Control of Vomition The complex, almost sterotypical set of activities that culminate in vomiting suggest that control is central, which indeed has been shown to be true. Within the brainstem are two anatomically and functionally distinct units that control vomiting: Bilateral vomition centers in the reticular formation of the medulla integrate signals from a large number of outlying sources and their excitement is ultimately what triggers vomition. Electric stimulation of these centers induces vomiting, while destruction of the vomition centers renders animals very resistant to emetic drugs. The vomition centers receive afferent signals from at least four major sources: * The chemoreceptor trigger zone (see below) * Visceral afferents from the gastrointestinal tract (vagus or sympathetic nerves) these signals inform the brain of such conditions as gastrointestinal distention (a very potent stimulus for vomition) and mucosal irritation. * Visceral afferents from outside the gastrointestinal tract - this includes signals from bile ducts, peritoneum, heart and a variety of other organs. These inputs to the vomition center help explain how, for example, a stone in the common bile duct can result in vomiting. * Afferents from extramedullary centers in the brain - it is clear that certain psychic stimuli (odors, fear), vestibular disturbances (motion sickness) and cerebral trauma can result in vomition. The chemoreceptor trigger zone is a bilateral set of centers in the brainstem lying under the floor of the fourth ventricle. Electrical stimulation of these centers does not induce vomiting, but application of emetic drugs does - if and only if the vomition centers are intact. The chemoreceptor trigger zones function as emetic chemoreceptors for the vomition centers - chemical abnormalities in the body (e.g. emetic drugs, uremia, hypoxia and diabetic ketoacidosis) are sensed by these centers, which then send excitatory signs to the vomition centers. Many of the antiemetic drugs act at the level of the chemoreceptor trigger zone. To summarize, two basic sets of pathways - one neural and one humoral - lead to activation of centers in the brain that initiate and control vomition. Think of the vomition centers as commander in chief of vomition, who makes the ultimate decision. This decision is based on input from a battery of advisors, among whom the chemoreceptor trigger zone has considerable influence. This straighforward picture is almost certainly oversimplified and flawed in some details, but helps to explain much of the physiology and pharmacology of vomition. Causes and Consequences of Vomiting The myriad causes of vomiting are left as an exercise - come up with a list based on personal experience and your understanding of the control of vomition. An important point, however, is that many cases of vomiting are due to diseases outside of the gastrointestinal tract. Simple vomiting rarely causes problems, but on occasion, can lead to such serious consequences as aspiration pneumonia. Additionally, severe or repetitive vomition results in disturbances in acid-base balance, dehydration and electrolyte depletion. In such cases, the goal is to rapidly establish a definitive diagnosis of the underlying disease so that specific therapy can be instituted. This is often not easy and in many cases, it is advantageous to administer antiemetic drugs in order to suppress vomition and reduce its sequelae. The Gastrointestinal Barrier -----------------------------------------------------------------------The gastrointestinal mucosa forms a barrier between the body and a lumenal environment which not only contains nutrients, but is laden with potentially hostile microorganisms and toxins. The challenge is to allow efficient transport of nutrients across the epithelium while rigorously excluding passage of harmful molecules and organisms into the animal. The exclusionary properties of the gastric and intestinal mucosa are referred to as the "gastrointestinal barrier". It is clear that a number of primary gastrointestinal diseases lead to disruption of the mucosal barrier, allowing escalation to systemic disease. It is equally clear that many systemic disease processes result in damage to the gastrointestinal barrier, thereby adding further insult to an already compromised system. Understanding the nature of the barrier can assist in predicting such events and aid in prophylactic or active therapies. The gastrointestinal barrier is often discussed as having two components: * The intrinsic barrier is composed of the epithelial cells lining the digestive tube and the tight junctions that tie them together. * The extrinsic barrier consists of secretions and other influences that are not physically part of the epithelium, but which affect the epithelial cells and maintain their barrier function. The Intrinsic Gastrointestinal Barrier The alimentary canal is lined by sheets of epithelial cells that form the defining structure of the mucosa. With few exceptions, epithelial cells in the stomach and intestines are circumferentially tied to one another by tight junctions, which seal the paracellular spaces and thereby establish the basic gastrointestinal barrier. Throughout the digestive tube, maintenance of an intact epithelium is thus critical to the integrity of the barrier. In general, toxins and microorganisms that are able to breach the single layer of epithelial cells have unimpeded access to the the systemic circulation. As might be anticipated, there is diversity among different types of epithelial cells in specific barrier functions. For example, the apical plasma membranes of gastric parietal and chief cells have atypically low permeability to protons, which aids in preventing damage due to back diffusion of acid into the cells. Small intestinal epithelial cells lack this specialized ability and thus are much more susceptible to acid-induced damage. Tight junctions encircling gastrointestinal epithelial cells are a critical component of the intrinsic barrier. These structures used to be viewed as passive structures akin to welds, but recent studies indicate that they are much more dynamic than previously thought, and their permeability may be regulated by a number of factors that affect the epithelial cells. The gastrointestinal epithelium is populated by a variety of functionally-mature cells derived from proliferation of stem cells. Most of the mature epithelial cells, including mucous cells in the stomach and absorptive cells in the small intestine, show rapid turnover rates, and die within only a few days after their formation. Maintenance of epithelial integrity thus requires a precise balance between cell proliferation and cell death. Stem cells that support continual replenishment of gastrointestinal epithelium reside in the middle of the gastric pits and within the crypts of the small and large intestine. Epithelial cell dynamics of the small intestine have been particularly well studied. These stem cells proliferate continually to supply cells that then differentiate into absorptive enterocytes, mucus-secreting goblet cells, enteroendocrine cells and Paneth cells. Except for Paneth cells, which remain in the crypts, the other cells differentiate into their mature forms as they migrate up from the crypts to replace cells extruded from the tips of the villi. This migration takes approximately 3 to 6 days. The Extrinsic Gastrointestinal Barrier Mucus and Bicarbonate The entire gastrointestinal epithelium is coated with mucus, which is synthesized by cells that form part of the epithelium. Mucus serves an important role in mitigating shear stresses on the epithelium and contributes to barrier function in several ways. The abundant carbohydrates on mucin molecules bind to bacteria, which aids in preventing epithelial colonization and, by causing aggregation, accelerates clearance. Diffusion of hydrophilic molecules is considerably lower in mucus than in aqueous solution, which is thought to retard diffusion of a variety of damaging chemicals, including gastric acid, to the epithelial surface. In addition to being coated with a mucus layer, gastric and duodenal epithelial cells secrete bicarbonate ion on their apical faces. This serves to maintain a neutral pH along the epithelial plasma membrane, even though highly acidic conditions exists in the lumen. Hormones and Cytokines Normal proliferation of gastric and intestinal epithelial cells, as well as proliferation in response to such injury as ulceration, is known to be affected by a large number of endocrine and paracrine factors. Several of the enteric hormones are known to enhance rates of proliferation. Different forms of injury to the epithelium can lead to either enhanced or suppressed rates of cell proliferation. For example, it has been demonstrated that resection of a portion of the canine small intestine is followed by epithelial cell hyperplasia and increased villous length in animals fed orally. Animals fed parenterally failed to show the same compensatory hyperplasia, indicating that, among other factors, local nutrients play an important role in cell dynamics. Prostaglandins, particularly prostaglandin E2 and prostacyclin, have long been known to have "cytoprotective" effects on the gastrointestinal epithelium. A common clinical correlate in many mammals is that use of aspirin and other non-steroidal antiinflammatory drugs (NSAIDs) which inhibit prostaglandin synthesis is commonly associated with gastric erosions and ulcers. Dogs are particularly sensitive to this side effect. Prostaglandins are synthesized within the mucosa from arachidonic acid, through the action of cyclooxygenases. Their cytoprotective effect appears to result from a complex ability to stimulate mucosal mucus and bicarbonate secretion, to increase mucosal blood flow and, particularly in the stomach, to limit back diffusion of acid into the epithelium. Considerable effort is underway to develop NSAIDs that fail to inhibit mucosal prostaglandin synthesis. Two peptides that have received attention for their potential role in barrier maintenance are epidermal growth factor (EGF) and transforming growth factor-alpha (TGF-alpha). EGF is secreted in saliva and from duodenal glands, while TGF-alpha is produced by gastric epithelial cells. Both peptides bind to a common receptor and stimulate epithelial cell proliferation. In the stomach, they also enhance mucus secretion and inhibit acid production. Other cytokines such as fibroblast growth factor and hepatocyte growth factor have been shown to enhance healing of gastrointestinal ulcers in experimental models. Trefoil proteins are a family of small peptides that are are secreted abundantly by goblet cells in the gastric and intestinal mucosa, and coat the apical face of the epithelial cells. Their distinctive molecular structure appears to render them resistant to proteolytic destruction. A number of studies have demonstrated that trefoil peptides play an important role in mucosal integrity, repair of lesions, and in limiting epithelial cell proliferation. They have been shown to protect the epithelium from a broad range of toxic chemicals and drugs. Trefoil proteins also appear to be a central player in the restitution phase of epithelial damage repair, where epithelial cells flatten and migrate from the wound edge to cover denuded areas. Mice with targeted deletions in trefoil genes showed exaggerated responses to mild chemical injury and delayed mucosal healing. Another molecule that plays a crucial role in mucosal integrity and barrier function is nitric oxide (NO). Paradoxically, NO also contributes to mucosal injury in a number of digestive diseases. This molecule is synthesized from arginine through the action of one of three isoforms of nitric oxide synthease (NOS). Much of the research in this area has focused on understanding the effects of applying NO donors such as glyceryltrinitrate or NOS inhibitors. In several models, NO donors significantly reduced the severity of mucosal injury induced by toxic chemicals (e.g. ethanol) or associated with ischemia and reperfusion. Similarly, healing of gastric ulcers in rats has been accelerated by application of NO donors. Another intriguing observation is that co-administration of NO donors and NSAIDs results in anti-inflammatory properties comparable to NSAIDs alone, but with less damage to the gastrointestinal mucosa. NOS inhibitors are under investigation for treatment of situations in which NO is overproduced and contributes to mucosal injury. Antibiotic Peptides and Antibodies An important part of barrier function is to prevent transit of bacteria from the lumen through the epithelium. Paneth cells are epithelial granulocytes located in small intestinal crypts of many mammals. They synthesize and secrete several antimicrobial peptides, chief among them isoforms of alpha-defensins known also as cryptdins ("crypt defensin"). These peptides have antimicrobial activity against of number of potential pathogens, including several genera of bacteria, some yeasts and Giardia trophozoites. Their mechanism of action is likely similar to neutrophilic alpha-defensins, which permeabilize target cell membranes. In addition to non-specific antimicrobial molecules, barrier function is supported by the gastrointestinal immune system. One facet of this defense systems is that much of the epithelium is bathed in secretory immunoglobulin A. This class of antibody is secreted from subepithelial plasma cells and transcytosed across the epithelium into the lumen. Lumenal IgA provides an antigenic barrier by binding bacteria and other antigens. This barrier function is specific for particular antigens and requires previous exposure for development of the response. Disruption of Barrier Function Despite its robust and multi-faceted nature, the gastrointestinal barrier can be breached. Local infections by bacteria and virus, exposure to toxins or physical insults, and a variety of systemic diseases lead to its disruption. Such problems can be mild and readily repaired, or massive and fatal. The micrographs below depict severe disruption of the barrier. On the left is mucosa from a normal canine small intestine, with large villi covered by intact epithelium extended into the lumen. The image on the right (same magnification) shows small intestinal mucosa from a dog that died of Salmonella enteritis - note the totally denuded epithelium and destruction of villi. Picture 48XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Ischemia and Reperfusion Injury Damage to the gastrointestinal barrier due to ischemia and reperfusion injury is a common and serious condition. Ischemia occurs when blood flow is insufficient to deliver an amount of oxygen and nutrients necessary for maintenance of cell integrity. Reperfusion injury occurs when blood flow is restored to ischemic tissue. Gastrointestinal ischemia results from two fundamental types of disorders, both of which can compromise the epithelial barrier: * Non-occlusive ischemia results from systemic conditions such as circulatory shock, sepsis or cardiac insufficiency. * Occlusive ischemia refers to conditions that directly disrupt gastrointestinal blood flow, such as strangulation, volvulus or thromboembolism. Reperfusion injury to the gastrointestinal wall, especially the mucosa, is thought to be due primarily to generation of reactive oxygen species, including superoxide, hydrogen peroxide and hydroxyl radicals. These oxidants are generated within the mucosa and also in the numerous local leukocytes activated during the course of ischemia. Oxygen-derived free radicals generated during reperfusion initiate a series of events that causes mucosal damage and disruption of the barrier. They directly damage cell membranes by forming lipid peroxides, which also leads to production of a number of inflammatory mediators derived from phospholipids (e.g. platelet-activiating factor and leukotrienes). These proinflammatory agents function as chemoattractants for neutrophils, which migrate into the mucosa, release their own reactive oxygen metabolites and cause further damage to the intrinsic epithelial barrier. An initially minor effect from ischemia is thus amplified into very significant damage to barrier function. Additinally, the inflammatory mediators generated in the gastrointestinal tract can harm distant tissues, leading to systemic disease. The observed effects of ischemia-reperfusion injury range from increased vascular permeability and consequent subepithelial edema, to massive loss of epithelial cells and villi. Even relatively mild damage to the epithelium disrupts barrier function and can lead to translocation of bacteria and toxins from the lumen to the systemic circulation. A number of treatments are under development and testing to prevent this cascade of damage, including application of antioxidants such as superoxide dismutase and use of drugs such as platelet-activating factor antagonists to block the effect of inflammatory mediators. Neutrophils and Mucosal Injury Diverse insults to the intestinal mucosa, including infectious processes, ischemia and damaging chemicals, promote infiltration of neutrophils. This common endpoint results because many types of injuries lead to local production of neutrophil chemoattractants such as leukotrienes, interleukins and activated complement components. In response to chemoattractants, neutrophils migrate our of capillaries, infiltrate the subepithelial mucosa and often transmigrate through the gastric or intestinal epithelium. In crossing the epithelium, neutrophils must break junctional complexes between epithelial cells. This "impalement" through tight junctions necessarily causes transient increases in permiability. When the insult is minor, the junctions reseal quickly, but transmigration of large numbers of neutrophils induces significant damage to barrier function. In addition to physically disrupting epithelial connections, infiltrating neutrophils themselves release reactive oxygen species and proteases that accentuate the damage to the epithelium and microvasculature. Blocking or suppressing the infiltration of neutrophils with such treatments as antibodies to integrins and other cell adhesion molecules may aid in protecting the mucosal barrier, but would likely have to be administered very early in the course of disease. Effects of Stress Stress comes in a myriad of forms and is an integral part of all illness and trauma. The stress response involves modulation of literally dozens of hormones and cytokines, as well as significant effects on neurotransmission. However, the foremost effect of stress on the gastrointestinal tract is to decrease mucosal blood flow and thereby compromise the integrity of the mucosal barrier. Among other things, reduced mucosal blood flow suppresses production of mucus and limits the ability to remove back diffusing protons. As a consequence, significant stress is almost always associated with mucosal erosions, particularly in the stomach. A majority of these lesions are subclinical, but gastrointestinal hemorrhage and sepsis are not infrequent consequences. Restitution and Healing After Injury The critical first task following disruption of the gastrointestinal epithelium is to cover the denuded area and re-establish the intrinsic barrier. This rapid restoration of epithelium is accomplished by a process called restitution - epithelial cells adjacent to the defect flatten and migrate over the exposed basement membrane. In the small intestine, this process is aided by a rapid contraction and shortening of the affected villi, which reduces the area of basement membrane that must be covered. Restitution provides a rapid mechanism for covering a defect in the barrier and does not involve proliferation of epithelial cells. It results in an area that, while protected, is not physiologically functional. Healing requires that the epithelial cells on the margins of the defect proliferate, differentiate and migrate into the damaged area to restore the normal cellular architecture and function. Restitution has been shown to be stimulated by a number of mostly paracrine regulators. Local prostaglandins and trefoil proteins are clearly involved in this process, and suppression of their production significantly delays restitution. Another group of molecules involved in restitution is the polyamines such as spermine, spermidine and putrescine. These molecules are present in many diets and also synthesized by the gastrointestinal mucosa. Enteral administration of polyamines has been shown in experimental models to accelerate restitution and healing of mucosal lesions. The Liver: Introduction and Index -----------------------------------------------------------------------The liver is the largest gland in the body and performs an astonishingly large number of tasks that impact all body systems. One consequence of this complexity is that hepatic disease has widespread effects on virtually all other organ systems. At the risk of losing sight of the forest by focusing on the trees, we will focus on three fundamental roles of the liver: * Vascular functions, including formation of lymph and the hepatic phagocytic system. * Metabolic achievements in control of synthesis and utilization of carbohydrates, lipids and proteins. * Secretory and excretory functions, particularly with respect to the synthesis of secretion of bile. The latter is the only one of the three that directly affects digestion - the liver, through its biliary tract, secretes bile acids into the small intestine where they assume a critical role in the digestion and absorption of dietary lipids. However, understanding the vascular and metabolic functions of the liver is critical to appreciating the gland as a whole. Core concepts in hepatic physiology are presented as the following topics: * * * * * Architecture of the liver and biliary tract Physiology of the hepatic vascular system Secretion of bile and the role of bile acids in digestion Biliary excretion of waste products: elimination of bilirubin Metabolic functions of the liver Advanced and supplemental topics related to physiology of the liver: * * * * * * * * Histology of the Liver and Biliary System Liver and Hepatocytes Regeneration of the Liver Bile and the Biliary System Gallstones (Cholelithiasis) Neonatal Jaundice Do You Enjoy Eating Eggs? The Role of Bile Acids as Hormones Architecture of the Liver and Biliary Tract -----------------------------------------------------------------------The liver lies in the abdominal cavity, in contact with diaphragm. As seen in image to the right of a mouse liver, its mass is divided into several lobes, the number and size of which vary among species. In most mammals, a greenish sac - the gall bladder - is seen attached to the liver and careful examination will reveal the common bile duct, which delivers bile from the liver and gall bladder into the duodenum. Understanding function and dysfunction of the liver, more than most other organs, depends on understanding its structure. The major aspects of hepatic structure that require detailed attention include: * The hepatic vascular system, which has several unique characteristics relative to other organs * The biliary tree, which is a system of ducts that transports bile out of the liver into the small intestine * The three dimensional arrangements of the liver cells, or hepatocytes and their association with the vascular and biliary systems. The Hepatic Vascular System The circulatory system of the liver is unlike that seen in any other organ. Of great importance is the fact that a majority of the liver's blood supply is venous blood! The pattern of blood flow in the liver can be summarized as follows: * Roughly 75% of the blood entering the liver is venous blood from the portal vein. Importantly, all of the venous blood returning from the small intestine, stomach, pancreas and spleen converges into the portal vein. One consequence of this is that the liver gets "first pickings" of everything absorbed in the small intestine, which, as we will see, is where virtually all nutrients are absorbed. * The remaining 25% of the blood supply to the liver is arterial blood from the hepatic artery. * Terminal branches of the hepatic portal vein and hepatic artery empty together and mix as they enter sinusoids in the liver. Sinusoids are distensible vascular channels lined with highly fenestrated or "holey" endothelial cells and bounded circumferentially by hepatocytes. As blood flows through the sinusoids, a considerable amount of plasma is filtered into the space between endothelium and hepatocytes (the "space of Disse"), providing a major fraction of the body's lymph. * Blood flows through the sinusoids and empties into the central vein of each lobule. * Central veins coalesce into hepatic veins, which leave the liver and empty into the vena cava. The Biliary System The biliary system is a series of channels and ducts that conveys bile - a secretory and excretory product of hepatocytes - from the liver into the lumen of the small intestine. Hepatocytes are arranged in "plates" with their apical surfaces facing and surrounding the sinusoids. The basal faces of adjoining hepatocytes are welded together by junctional complexes to form canaliculi, the first channel in the biliary system. A bile canaliculus is not a duct, but rather, the dilated intercellular space between adjacent hepatocytes. Hepatocytes secrete bile into the canaliculi, and those secretions flow parallel to the sinusoids, but in the opposite direction that blood flows. At the ends of the canaliculi, bile flows into bile ducts, which are true ducts lined with epithelial cells. Bile ducts thus begin in very close proximity to the terminal branches of the portal vein and hepatic artery, and this group of structures is an easily recognized and important landmark seen in histologic sections of liver - the grouping of bile duct, hepatic arteriole and portal venule is called a portal triad. Small bile ducts, or ductules, anastomose into larger and larger ducts, eventually forming the common bile duct, which dumps bile into the duodenum. A sphincter known as the sphinter of Oddi is present around the common bile duct as it enters the intestine. The gall bladder is another important structure in the biliary system of many species. This is a sac-like structure adhering to the liver which has a duct (cystic duct) that leads directly into the common bile duct. During periods of time when bile is not flowing into the intestine, it is diverted into the gall bladder, where it is dehydrated and stored until needed. Architecture of the Hepatic Parenchyma The liver is covered with a connective tissue capsule that branches and extends throughout the substance of the liver as septae. This connective tissue tree provides a scaffolding of support and the highway which along which afferent blood vessels, lymphatic vessels and bile ducts traverse the liver. Additionally, the sheets of connective tissue divide the parenchyma of the liver into very small units called lobules. The hepatic lobule is the structural unit of the liver. It consists of a roughly hexagonal arrangement of plates of hepatocytes radiating outward from a central vein in the center. At the vertices of the lobule are regularly distributed portal triads, containing a bile duct and a terminal branch of the hepatic artery and portal vein. Lobules are particularly easy to see in pig liver because in that species they are well deliniated by connective tissue septae that invaginate from the capsule. Additional information on liver structure is presented in the sections on hepatic histology. Physiology of the Hepatic Vascular System -----------------------------------------------------------------------Hepatic Blood Volume and Reservoir Function The liver receives approximately 30% of resting cardiac output and is therefore a very vascular organ. The hepatic vascular system is dynamic, meaning that it has considerable ability to both store and release blood - it functions as a reservoir within the general circulation. In the normal situation, 10-15% of the total blood volume is in the liver, with roughly 60% of that in the sinusoids. When blood is lost, the liver dynamically adjusts its blood volume and can eject enough blood to compensate for a moderate amount of hemorrhage. Conversely, when vascular volume is acutely increased, as when fluids are rapidly infused, the hepatic blood volume expands, providing a buffer against acute increases in systemic blood volume. Formation of Lymph in the Liver Approximately half of the lymph formed in the body is formed in the liver. Due to the large pores or fenestrations in sinusoidal endothelial cells, fluid and proteins in blood flow freely into the space between the endothelium and hepatocytes (the "space of Disse"), forming lymph. Lymph flows through the space of Disse to collect in small lymphatic capillaries associated with portal triads (the reason they are not called portal tetrads is because these lymphatic vessels are virtually impossible to identify in standard histologic sections), and from there in the systemic lymphatic system. As you might expect, if pressure in the sinusoids increases much above normal, there is a corresponding increase in the rate of lymph production. In severe cases the liver literally sweats lymph, which accumulates in the abdominal cavity as ascitic fluid. What lesions can you envision that would raise blood pressure in sinusoids, resulting in production of ascites ? The Hepatic Phagocytic System The liver is host to a very important part of the phagocytic system. Lurking in the sinusoids are large numbers of a type of tissue macrophage known as the Kupffer cell.Kupffer cells are actively phagocytic and represent the main cellular system for removal of particulate materials and microbes from the circulation. The image below is a lightly stained section of liver from a mouse that was injected intravenously with a very small quantity of India ink - Kupffer cells are clearly visible throughout the section because they have phagocytosed the ink particles and appear dark black. Their location just downstream from the portal vein allows Kupffer cells to efficiently scavenge bacteria that get into portal venous blood through breaks in the intestinal epithelium, thus preventing invasion of the systemic circulation. Secretion of Bile and the Role of Bile Acids In Digestion -----------------------------------------------------------------------Bile is a complex fluid containing water, electrolytes and a battery of organic molecules including bile acids, cholesterol, phospholipids and bilirubin that flows through the biliary tract into the small intestine. There are two fundamentally important functions of bile in all species: * Bile contains bile acids, which are critical for digestion and absorption of fats and fat-soluble vitamins in the small intestine. * Many waste products are eliminated from the body by secretion into bile and elimination in feces. Adult humans produce 400 to 800 ml of bile daily, and other animals proportionately similar amounts. The secretion of bile can be considered to occur in two stages: * Initially, hepatocytes secrete bile into canaliculi, from which it flows into bile ducts. This hepatic bile contains large quantities of bile acids, cholesterol and other organic molecules. * As bile flows through the bile ducts it is modified by addition of a watery, bicarbonate-rich secretion from ductal epithelial cells. In species with a gallbladder (man and most domestic animals except horses and rats), further modification of bile occurs in that organ. The gall bladder stores and concentrates bile during the fasting state. Typically, bile is concentrated five-fold in the gall bladder by absorption of water and small electrolytes - virtually all of the the organic molecules are retained. Secretion into bile is a major route for eliminating cholesterol. Free cholesterol is virtually insoluble in aqueous solutions, but in bile, it is made soluble by bile acids and lipids like lethicin. Gallstones, most of which are composed predominantly of cholesterol, result from processes that allow cholesterol to precipitate from solution in bile. Role of Bile Acids in Fat Digestion and Absorption Bile acids are derivatives of cholesterol synthesized in the hepatocyte. Cholesterol, ingested as part of the diet or derived from hepatic synthesis is converted into the bile acids cholic and chenodeoxycholic acids, which are then conjugated to an amino acid (glycine or taurine) to yield the conjugated form that is actively secreted into cannaliculi. Bile acids are facial amphipathic, that is, they contain both hydrophobic (lipid soluble) and polar (hydrophilic) faces. The cholesterol-derived portion of a bile acid has one face that is hydrophobic (that with methyl groups) and one that is hydrophilic (that with the hydroxyl groups); the amino acid conjugate is polar and hydrophilic. Their amphipathic nature enables bile acids to carry out two important functions: * Emulsification of lipid aggregates: Bile acids have detergent action on particles of dietary fat which causes fat globules to break down or be emulsified into minute, microscopic droplets. Emulsification is not digestion per se, but is of importance because it greatly increases the surface area of fat, making it available for digestion by lipases, which cannot access the inside of lipid droplets. * Solubilization and transport of lipids in an aqueous environment: Bile acids are lipid carriers and are able to solubilize many lipids by forming micelles - aggregates of lipids such as fatty acids, cholesterol and monoglycerides - that remain suspended in water. Bile acids are also critical for transport and absorption of the fat-soluble vitamins. Role of Bile Acids in Cholesterol Homeostasis Hepatic synthesis of bile acids accounts for the majority of cholesterol breakdown in the body. In humans, roughly 500 mg of cholesterol are converted to bile acids and eliminated in bile every day. This route for elimination of excess cholesterol is probably important in all animals, but particularly in situations of massive cholesterol ingestion. Interestingly, it has recently been demonstrated that bile acids participate in cholesterol metabolism by functioning as hormones that alter the transcription of the rate-limiting enzyme in cholesterol biosynthesis. Enterohepatic Recirculation Large amounts of bile acids are secreted into the intestine every day, but only relatively small quantities are lost from the body. This is because approximately 95% of the bile acids delivered to the duodenum are absorbed back into blood within the ileum. Venous blood from the ileum goes straight into the portal vein, and hence through the sinusoids of the liver. Hepatocytes extract bile acids very efficiently from sinusoidal blood, and little escapes the healthy liver into systemic circulation. Bile acids are then transported across the hepatocytes to be resecreted into canaliculi. The net effect of this enterohepatic recirculation is that each bile salt molecule is reused about 20 times, often two or three times during a single digestive phase. It should be noted that liver disease can dramatically alter this pattern of recirculation for instance, sick hepatocytes have decreased ability to extract bile acids from portal blood and damage to the canalicular system can result in escape of bile acids into the systemic circulation. Assay of systemic levels of bile acids is used clinically as a sensitive indicator of hepatic disease. Pattern and Control of Bile Secretion The flow of bile is lowest during fasting, and a majority of that is diverted into the gallbladder for concentration. When chyme from an ingested meal enters the small intestine, acid and partially digested fats and proteins stimulate secretion of cholecystokinin and secretin. As discussed previously, these enteric hormones have important effects on pancreatic exocrine secretion. They are both also important for secretion and flow of bile: * Cholecystokinin: The name of this hormone describes its effect on the biliary system - cholecysto = gallbladder and kinin = movement. The most potent stimulus for release of cholecystokinin is the presence of fat in the duodenum. Once released, it stimulates contractions of the gallbladder and common bile duct, resulting in delivery of bile into the gut. * Secretin: This hormone is secreted in response to acid in the duodenum. Its effect on the biliary system is very similar to what was seen in the pancreas - it simulates biliary duct cells to secrete bicarbonate and water, which expands the volume of bile and increases its flow out into the intestine. The processes of gallbladder filling and emptying described here can be visualized using an imaging technique called scintography. This procedure is utilized as a diagnostic aid in certain types of hepatobiliary disease. Biliary Excretion of Waste Products: Elimination of Bilirubin -----------------------------------------------------------------------The liver is well known to metabolize and excrete into bile many compounds and toxins, thus eliminating them (usually) from the body. Examples can be found among both endogenous molecules (steroid hormones, calcium) and exogenous compounds (many antibiotics and metabolities of drugs). A substantial number of these compounds are reabsorbed in the small intestine and ultimately eliminated by the kidney. One of the most important and clinically relevant examples of waste elimination via bile is that of bilirubin. Additionally, the mechanisms involved in elimination of bilirubin are similar to those used for elimination of many drugs and toxins. Bilirubin is a useless and toxic breakdown product of hemoglobin, which also means that it is generated in large quantities. In the time it takes you to read this sentence aloud, roughly 20 million of your red blood cells have died and roughly 5 quintillion (5 x 1015) molecules of hemoglobin are in need of disposal. The dead and damaged red blood cells are picked up by phagocytic cells throughout the body (including Kuppfer cells in the liver) and digested. The iron is precious and is efficiently recycled. The globin chains are protein and are catabolized and their components reused. However, hemoglobin also contains a porphyrin called heme that cannot be recycled and must be eliminated. Elimination of heme is accomplished in a series of steps: * Within the phagocytic cells, heme is converted through a series of steps into free bilirubin, which is released into plasma where it is carried around bound to albumin, itself a secretory product of the liver. * Free bilirubin is stripped off albumin and absorbed by - you guessed it hepatocytes. Within hepatocytes, free bilirubin is conjugated to either glucuronic acid or sulfate - it is then called conjugated bilirubin. * Conjugated bilirubin is secreted into the bile canaliculus as part of bile and thus delivered to the small intestine. Bacteria in the intestinal lumen metabolize bilirubin to a series of other compounds which are ultimately eliminated either in feces or, after reabsortion, in urine. The major metabolite of bilirubin in feces is sterobilin, which gives feces their characteristic brown color. If excessive quantities of either free or conjugated bilirubin accumulate in extracellular fluid, a yellow discoloration of the skin, sclera and mucous membranes is observed - this condition is called icterus or jaundice. Determining whether the excessive bilirubin is free or conjugated can aid in diagnosing the cause of the problem. Metabolic Functions of the Liver -----------------------------------------------------------------------Hepatocytes are metabolic superachievers in the body. They play critical roles in synthesizing molecules that are utilized elsewhere to support homeostasis, in converting molecules of one type to another, and in regulating energy balances. If you have taken a course in biochemistry, you probably spent most of that class studying metabolic pathways of the liver. At the risk of damning by faint praise, the major metabolic functions of the liver can be summarized into several major categories: Carbohydrate Metabolism It is critical for all animals to maintain concentrations of glucose in blood within a narrow, normal range. Maintainance of normal blood glucose levels over both short (hours) and long (days to weeks) periods of time is one particularly important function of the liver. Hepatocytes house many different metabolic pathways and employ dozens of enzymes that are alternatively turned on or off depending on whether blood levels of glucose are rising or falling out of the normal range. Two important examples of these abilities are: * Excess glucose entering the blood after a meal is rapidly taken up by the liver and sequestered as the large polymer, glycogen (a process called glycogenesis). Later, when blood concentrations of glucose begin to decline, the liver activates other pathways which lead to depolymerization of glycogen (glycogenolysis) and export of glucose back into the blood for transport to all other tissues. * When hepatic glycogen reserves become exhaused, as occurs when an animal has not eaten for several hours, do the hepatocytes give up? No! They recognize the problem and activate additional groups of enzymes that begin synthesizing glucose out of such things as amino acids and non-hexose carbohydrates (gluconeogenesis). The ability of the liver to synthesize this "new" glucose is of monumental importance to carnivores, which, at least in the wild, have diets virtually devoid of starch. Fat Metabolism Few aspects of lipid metabolism are unique to the liver, but many are carried out predominantly by the liver. Major examples of the role of the liver in fat metabolism include: * The liver is extremely active in oxidizing triglycerides to produce energy. The liver breaks down many more fatty acids that the hepatocytes need, and exports large quantities of acetoacetate into blood where it can be picked up and readily metabolized by other tissues. * A bulk of the lipoproteins are synthesized in the liver. * The liver is the major site for converting excess carbohydrates and proteins into fatty acids and triglyceride, which are then exported and stored in adipose tissue. * The liver synthesizes large quantities of cholesterol and phospholipids. Some of this is packaged with lipoproteins and made available to the rest of the body. The remainder is excreted in bile as cholesterol or after converstion to bile acids. Protein Metabolism The most critical aspects of protein metabolism that occur in the liver are: * Deamination and transamination of amino acids, followed by conversion of the nonnitrogenous part of those molecules to glucose or lipids. Several of the enzymes used in these pathways (for example, alanine and aspartate aminotransferases) are commonly assayed in serum to assess liver damage. * Removal of ammonia from the body by synthesis of urea. Ammonia is very toxic and if not rapidly and efficiently removed from the circulation, will result in central nervous system disease. A frequent cause of such hepatic encephalopathy in dogs and cats are malformations of the blood supply to the liver called portosystemic shunts. * Synthesis of non-essential amino acids. * Hepatocytes are responsible for synthesis of most of the plasma proteins. Albumin, the major plasma protein, is synthesized almost exclusively by the liver. Also, the liver synthesizes many of the clotting factors necessary for blood coagulation. Histology of the Liver -----------------------------------------------------------------------Looking at a section of liver is somewhat reminiscent of looking down out of an airplane at a suburban neighborhood. One sees a very regular, almost monotonous, collection of houses in blocks demarcated by roads, with a gas station or minimart apparent at almost every intersection. In the case of the liver, the roads are connective tissue septae which convey vascular and biliary traffic, and the clusters of houses are cord-like arrangements of hepatocytes, the parenchymal cell of the liver. Prior to embarking on the lessons below, it would be best to review the core section Architecture of the Liver and Biliary Tract. The lessons below are somewhat graphics intensive and will be disappointing if your browser is not Java-enabled or your monitor is not capable of high resolution color. Summary of Lesson Link Sheets of connective tissue divide the liver into thousands of small units called lobules. A lobule is roughly hexagonal in shape, with portal triads at the vertices and a central vein in the middle. The lobule is the structural unit of the liver and rather easy to observe. In contrast, the hepatic acinus is more difficult to visualize, but represents a unit that is of more relevance to hepatic function because it is oriented around the afferent vascular system. The parenchymal cells of the liver are hepatocytes. These polygonal cells are joined to one another in anastomosing plates, with borders that face either the sinusoids or adjacent hepatocytes. The ultrastructure appearance of hepatocytes reflects their function as metabolic superstars, with abundant rough and smooth endoplasmic reticulum, and Golgi membranes. Glycogen granules and vesicles containing very low density lipoproteins are readily observed. Hepatocytes make contact with blood in sinusoids, which are distensible vascular channels lined with highly fenestrated endothelial cells and populated with phagocytic Kupffer cells. The space between endothelium and hepatocytes is called the Space of Disse which collects lymph for delivery to lymphatic capillaries. Bile originates as secretions from the basal surface of hepatocytes, which collect in channels called canaliculi. These secretions flow toward the periphery of lobules and into bile ductules and interlobular bile ducts, ultimately collecting in the hepatic duct outside the liver. The hepatic duct is continuous with the common bile duct, which delivers bile into the duodenum. In most species, bile is diverted through the cystic duct into the gall bladder. The columnar epithelium of the gall bladder is devoted largely to absorption of water and electrolytes. Falta de aqui en adelante-----------------------------------------------------------------------The Pancreas: Introduction and Index -----------------------------------------------------------------------As chyme floods into the small intestine from the stomach, two things must happen: * acid must be quickly and efficiently neutralized to prevent damage to the duodenal mucosa * macromolecular nutrients - proteins, fats and starch - must be broken down much further before their constitutents can be absorbed through the mucosa into blood The pancreas plays a vital role in accomplishing both of these objectives, so vital in fact that insufficient exocrine secretion by the pancreas leads to starvation, even if the animal is consuming adequate quantities of high quality food. In addition to its role as an exocrine organ, the pancreas is also an endocrine organ and the major hormones it secretes - insulin and glucagon - play a vital role in carbohydrate and lipid metabolism. They are, for example, absolutely necessary for maintaining normal blood concentrations of glucose. Core concepts in physiology of the exocrine pancreas are presented as the following topics: * * * Gross and microscopic anatomy of the pancreas Exocrine secretions of the pancreas Control of pancreatic exocrine secretion Advanced and supplemental topics related to physiology of the pancreas: * Histology of the pancreas Gross and Microscopic Anatomy of the Pancreas -----------------------------------------------------------------------The pancreas is a elongated organ, light tan or pinkish in color, that lies in close proximity to the duodenum. It is covered with a very thin connective tissue capsule which extends inward as septa, partitioning the gland into lobules. The image to the right shows a portion of a canine pancreas nestled next to the duodenum. The bulk of the pancreas is composed of pancreatic exocrine cells and their associated ducts. Embedded within this exocrine tissue are roughly one million small clusters of cells called the Islets of Langerhans, which are the endocrine cells of the pancreas and secrete insulin, glucagon and several other hormones. In the histologic image of an equine pancreas seen below, a single islet is seen in the middle as a large, pale-staining cluster of cells. All of the surrounding tissue is exocrine. Pancreatic exocrine cells are arranged in grape-like clusters called acini. The exocrine cells themselves are packed with membrane-bound secretory granules which contain digestive enzymes that are exocytosed into the lumen of the acinus. From there these secretions flow into larger and larger, intralobular ducts, which eventually coalesce into the main pancreatic duct which drains directly into the duodenum. The lumen of an acinus communicates directly with intralobular ducts, which coalesce into interlobular ducts and then into the major pancreatic duct. Epithelial cells of the the intralobular ducts actually project "back" into the lumen of the acinus, where they are called centroacinar cells. The anatomy of the main pancreatic duct varies among species. In some animals, two ducts enter the duodenum rather than a single duct. In some species, the main pancreatic duct fuses with the common bile duct just before its entry into the duodenum. Exocrine Secretions of the Pancreas -----------------------------------------------------------------------Pancreatic juice is composed of two secretory products critical to proper digestion: digestive enzymes and bicarbonate. The enzymes are synthesized and secreted from the exocrine ascinar cells, whereas bicarbonate is secreted from the epithelial cells lining small pancreatic ducts. Digestive Enzymes The pancreas secretes a magnificent battery of enzymes that collectively have the capacity to reduce virtually all digestible macromolecules into forms that are capable of, or nearly capable of being absorbed. Three major groups of enzymes are critical to efficient digestion: Proteases Digestion of proteins is initiated by pepsin in the stomach, but the bulk of protein digestion is due to the pancreatic proteases. Several proteases are synthesized in the pancreas and secreted into the lumen of the small intestine. The two major pancreatic proteases are trypsin and chymotrypsin, which are synthesized and packaged into secretory vesicles as an the inactive proenzymes trypsinogen and chymotrypsinogen. As you might anticipate, proteases are rather dangerous enzymes to have in cells, and packaging of an inactive precursor is a way for the cells to safely handle these enzymes. The secretory vesicles also contain a trypsin inhibitor which serves as an additional safeguard should some of the trypsinogen be activated to trypsin; following exocytosis this inhibitor is diluted out and becomes ineffective - the pin is out of the grenade. Once trypsinogen and chymotrypsinogen are released into the lumen of the small intestine, they must be converted into their active forms in order to digest proteins. Trypsinogen is activated by the enzyme enterokinase, which is embedded in the intestinal mucosa. Once trypsin is formed it activates chymotrypsinogen, as well as additional molecules of trypsinogen. The net result is a rather explosive appearance of active protease once the pancreatic secretions reach the small intestine. Trypsin and chymotrypsin digest proteins into peptides and peptides into smaller peptides, but they cannot digest proteins and peptides to single amino acids. Some of the other proteases from the pancreas, for instance carboxypeptidase, have that ability, but the final digestion of peptides into amino acids is largely the effect of peptidases in small intestinal epithelial cells. More on this later. Pancreatic Lipase The major form of dietary fat is triglyceride, or neutral lipid. A triglyceride molecule cannot be directly absorbed across the intestinal mucosa. Rather, it must first be digested into a 2-monoglyceride and two free fatty acids. The enzyme that performs this hydrolysis is pancreatic lipase, which is delivered into the lumen of the gut as a constituent of pancreatic juice. Sufficient quantities of bile salts must also be present in the lumen of the intestine in order for lipase to efficiently digest dietary triglyceride and for the resulting fatty acids and monoglyceride to be absorbed. This means that normal digestion and absorption of dietary fat is critically dependent on secretions from both the pancreas and liver. Pancreatic lipase has recently been in the limelight as a target for management of obesity. The drug orlistat (Xenical) is a pancreatic lipase inhibitor that interferes with digestion of triglyceride and thereby reduces absorption of dietary fat. Clinical trials support the contention that inhibiting lipase can lead to significant reductions in body weight in some patients. Amylase The major dietary carbohydrate for many species is starch, a storage form of glucose in plants. Amylase is the enzyme that hydrolyses starch to maltose (a glucose-glucose disaccharide), as well as the trisaccharide maltotriose and small branchpoints fragments called limit dextrins. The major source of amylase in all species is pancreatic secretions, although amylase is also present in saliva of some animals, including man. Other Pancreatic Enzymes In addition to the proteases, lipase and amylase, the pancreas produces a host of other digestive enzymes, including ribonuclease, deoxyribonuclease, gelatinase and elastase. Bicarbonate and Water Epithelial cells in pancreatic ducts are the source of the bicarbonate and water secreted by the pancreas. The mechanism underlying bicarbonate secretion is essentially the same as for acid secretion parietal cells and is dependent on the enzyme carbonic anhydrase. In pancreatic duct cells, the bicarbonate is secreted into the lumen of the duct and hence into pancreatic juice. Control of Pancreatic Exocrine Secretion -----------------------------------------------------------------------As you might expect, secretion from the exocrine pancreas is regulated by both neural and endocrine controls. During interdigestive periods, very little secretion takes place, but as food enters the stomach and, a little later, chyme flows into the small intestine, pancreatic secretion is strongly stimulated. Like the stomach, the pancreas is innervated by the vagus nerve, which applies a low level stimulus to secretion in response to anticipation of a meal. However, the most important stimuli for pancreatic secretion comes from three hormones secreted by the enteric endocrine system: * Cholecystokinin: This hormone is synthesized and secreted by enteric endocrine cells located in the duodenum. Its secretion is strongly stimulated by the presence of partially digested proteins and fats in the small intestine. As chyme floods into the small intestine, cholecystokinin is released into blood and binds to receptors on pancreatic acinar cells, ordering them to secrete large quantities of digestive enzymes. * Secretin: This hormone is also a product of endocrinocytes located in the epithelium of the proximal small intestine. Secretin is secreted (!) in response to acid in the duodenum, which of course occurs when acid-laden chyme from the stomach flows through the pylorus. The predominant effect of secretin on the pancreas is to stimulate duct cells to secrete water and bicarbonate. As soon as this occurs, the enyzmes secreted by the acinar cells are flushed out of the pancreas, through the pancreatic duct into the duodenum. * Gastrin: This hormone, which is very similar to cholecystokinin, is secreted in large amounts by the stomach in response to gastric distention and irritation. In addition to stimulating acid secretion by the parietal cell, gastrin stimulates pancreatic acinar cells to secrete digestive enzymes. Stop and think about this for a minute - control of pancreatic secretion makes perfect sense. Pancreatic secretions contain enzymes which are needed to digest proteins, starch and triglyceride. When these substances enter stomach, and especially the small intestine, they stimulate release of gastrin and cholecystokinin, which in turn stimulate secretion of the enzymes of destruction. Pancreatic secretions are also the major mechanism for neutralizing gastric acid in the small intestine. When acid enters the small gut, it stimulates secretin to be released, and the effect of this hormone is to stimulate secretion of lots of bicarbonate. As proteins and fats are digested and absorbed, and acid is neutralized, the stimuli for cholecystokinin and secretin secretion disappear and pancreatic secretion falls off. Histology of the Pancreas -----------------------------------------------------------------------The structure of the pancreas is dominated by the fact that it is a dual function organ with both exocrine and endocrine cell types. The vast bulk of the pancreas is composed of exocrine tissue, and secretions from those cells flow into a series ducts for ultimate delivery into the duodenum. Prior to embarking on the lessons below, it would be best to review the core section Gross and Microscopic Anatomy of the Pancreas. The lessons below are somewhat graphics intensive and will be disappointing if your browser is not Java-enabled. Summary of Lesson Link The pancreas is divided into lobules by connective tissue septae. Lobules are composed largely of grape-like clusters of exocrine cells called acini, which secrete digestive enzymes. Exocrine secretions from acini flow successively through intercalated ducts, intralobular ducts, interlobular ducts and finally into the duodenum through the main pancreatic duct. Embedded within the pancreatic exocrine tissue are Islets of Langerhans, the endocrine component of the pancreas. Islets contain several cell types and are richly vascularized. The Small Intestine: Introduction and Index ------------------------------------------------------------------------ The small intestine is the portal for absorption of virtually all nutrients into blood. Accomplishing this transport entails breaking down large supramolecular aggregates into small molecules that can be transported across the epithelium. An exception to this statement is seen in herbivores, where large amounts of short chain fatty acids are absorbed at other sites. By the time ingesta reaches the small intestine, foodstuffs have been mechanically broken down and reduced to a liquid by mastication and grinding in the stomach. Once within the small intestine, these macromolecular aggregates are exposed to pancreatic enzymes and bile, which enables digestion to molecules capable or almost capable of being absorbed. The final stages of digestion occur on the surface of the small intestinal epithelium. The net effect of passage through the small intestine is absorption of most of the water and electrolytes (sodium, chloride, potassium) and essentially all dietary organic molecules (including glucose, amino acids and fatty acids). Through these activities, the small intestine not only provides nutrients to the body, but plays a critical role in water and acidbase balance. Core concepts in small intestinal physiology are presented as the following topics: * * * * * * * * * * Gross and microanatomy of the small intestine Villi, crypts and the life cycle of small intestinal enterocytes Small intestinal motility Overview of transport across the intestinal epithelium Secretion in the small intestine Absorption in the small intestine: General mechanisms Water and electrolytes Glucose and other monosaccharides Peptides and amino acids Lipids: triglyceride, monoglyceride, fatty acids Advanced and supplemental topics related to physiology of the small intestine: * * * * * Histology of the Small Intestine Absorption of Minerals and Metals Absorption of Vitamins Ontogeny of Small Intestinal Digestive and Absorptive Function The Three Compartment Model for Transport of Water Across Epithelium Gross and Microscopic Anatomy of the Small Intestine -----------------------------------------------------------------------The small intestine is the longest section of the digestive tube and consists of three segments forming a passage from the pylorus to the large intestine: * Duodenum: a short section that receives secretions from the pancreas and liver via the pancreatic and common bile ducts. * Jejunum: considered to be roughly 40% of the small gut in man, but closer to 90% in animals. * Ileum empties into the large intestine; considered to be about 60% of the intestine in man, but veterinary anatomists usually refer to it as being only the short terminal section of the small intestine. In most animals, the length of the small intestine is roughly 3.5 times body length - your small intestine, or that of a large dog, is about 6 meters in length. Although precise boundaries between these three segments of bowel are not observed grossly or microscopically, there are histologic differences among duodenum, jejunum and ileum. A bulk of the small intestine is suspended from the body wall by an extension of the peritoneum called the mesentery. As seen in the image to the right, blood vessels to and from the intestine lie between the two sheets of the mesentery. Lymphatic vessels are also present, but are not easy to discern grossly in normal specimens. It is within the small intestine that the final stages of enzymatic digestion occur, liberating small molecules capable of being absorbed. The small intestine is also the sole site in the digestive tube for absorption of amino acids and monosaccharides. Most lipids are also absorbed in this organ. All of this absorption and much of the enzymatic digestion takes place on the surface of small intestinal epithelial cells, and to accomodate these processes, a huge mucosal surface area is required. If the small intestine is viewed as a simple pipe, its lumenal surface area would be on the order of one half of a square meter. But in reality, the absorptive surface area of the small intestine is roughly 250 square meters - the size of a tennis court! How is this possible? At first glance, the structure of the small intestine is similar to other regions of the digestive tube, but the small intestine incorporates three features which account for its huge absorptive surface area: * Mucosal folds: the inner surface of the small intestine is not flat, but thrown into circular folds, which not only increase surface area, but aid in mixing the ingesta by acting as baffles. * Villi: the mucosa forms multitudes of projections which protrude into the lumen and are covered with epithelial cells. * Microvilli: the lumenal plasma membrane of absorptive epithelial cells is studded with densely-packed microvilli. The panels below depict the bulk of this surface area expansion, showing villi, epithelial cells that cover the villi and the microvilli of the epithelial cells. Note in the middle panel, a light micrograph, that the microvilli are visible and look something like a brush. For this reason, the microvillus border of intestinal epithelial cells is referred to as the "brush border". Most of the discussion on following pages focuses on enterocytes, the epithelial cells which mature into absorptive epithelial cells that cover the villi. These are the cells that take up and deliver into blood virtually all nutrients from the diet. However, two other major cell types populate the small intestinal epithelium: * Enteroendocrine cells which, as part of the enteric endocrine system sense the lumenal environment and secrete hormones such as cholecystokinin and gastrin into blood. * Goblet cells, which secrete a lubricating mucus into the intestinal lumen. Villi, Crypts and the Life Cycle of Small Intestinal Enterocytes -----------------------------------------------------------------------If examined closely, the lumenal surface of the small intestine appears similar to velvet due to its being covered by millions of small projections called villi which extend about 1 mm into the lumen. Villi are only the most obvious feature of the mucosa which houses a dynamic, self-renewing population of epithelial cells that includes secretory cells, endocrine cells and the mature absorptive epithelial cells which take up nutrients from the lumen and transport them into blood, fulfilling the basic function of the digestive system. Understanding how the small intestine functions requires looking at the structure of the mucosa in more detail. Epithelial Cell Dynamics The mucosa of small intestinal mucosa is arranged into two fundamental structures: Villi are projections into the lumen covered predominantly with mature, absorptive enterocytes, along with occasional mucus-secreting goblet cells. These cells live only for a few days, die and are shed into the lumen to become part of the ingesta to be digested and absorbed. That's right, we're all really cannibals. Crypts (of Lieberkuhn) are moat-like invaginations of the epithelium around the villi, and are lined largely with younger epithelial cells which are involved primarily in secretion. Toward the base of the crypts are stem cells, which continually divide and provide the source of all the epithelial cells in the crypts and on the villi. Small Intestinal Motility -----------------------------------------------------------------------Coordinated contractions of smooth muscle participate in several ways to facilitate digestion and absorption in the small intestine: * foodstuffs are mixed with digestive enzymes from the pancreas and bile salts from the biliary system * nutrient molecules in the lumen are constantly dispersed, allowing them to contact the epithelium where enzymatic digestion is completed and absorption occurs * chyme is moved down the digestive tube, making way for the next load and also eliminating undigestable, perhaps toxic substances In most animals, the small intestine cycles through two states, each of which is associated with distinctive patterns of motility: * Following a meal, when the lumen of the small intestine contains chyme, two types of motility predominate: segmentation contractions chop, mix and roll the chyme and peristalsis slowly propels it toward the large intestine. * The interdigestive state is seen between meals, when the lumen is largely devoid of contents. During such times, so-called housekeeping contractions propagate from the stomach through the entire small intestine, sweeping it clear of debris. This complex pattern of motility is also known as the migrating motor complex and is the cause of "growling". Motility in the small intestine, as in all parts of the digestive tube, is controlled predominantly by excitatatory and inhibitory signmals from the enteric nervous system. These local nervous signals are however modulated by inputs from the central nervous system, and a number of gastrointestinal hormones appear to affect intestinal motility to some degree. Overview of Transport Across the Intestinal Epithelium -----------------------------------------------------------------------There are two routes for transport across the epithelium of the gut: * * Across the plasma membrane of the epithelial cells (transcellular route) Across tight junctions between epithelial cells (paracellular route) Some molecules, water for instance, are transported by both routes. In contrast, the tight junctions are impermeable to large organic molecules from the diet (e.g. amino acids and glucose). Those types of molecules are transported exclusively by the transcellular route, and only because the plasma membrane of the absorptive enterocytes is equipped with transporter molecules that facilitate entry into and out of the cells. It is important to recognize that the epithelium of the gut is not a monotonous sheet of functionally identical cells. As ingesta travels through the intestine, it is sequentially exposed to regions having epithelia with very different characteristics. This diversity in function results from differences in phenotype of the enterocytes - that is, the number and type of transporter molecules they express in their plasma membrane and the structure of the tight junctions they form. Even within a given segment there are major differences in the type of transport that occurs - for example, cells in the crypts transport very differently than cells on the tips of villi. Within the intestine, there is a proximal to distal gradient in osmotic permiability. As you proceed down the tube, the effective pore size through the epithelium decreases. This means that the duodenum is much more "leaky" to water than the ileum and the ileum more leaky than the colon. Do not interpret this to mean that as you go down the tube, the ability to absorb water decreases! It means that water flows across the epithelium more "freely" in the proximal compared to distal gut because the effective pore size is larger. The distal intestine actually can absorb water better than the proximal gut. The observed differences in permiability to water across the epithelium is due almost entirely to differences in conductivity across the paracellular path - the takehome message is that tight junctions vary considerably in "tightness" along the length of the gut. Secretion in the Small Intestine -----------------------------------------------------------------------Large quantities of water are secreted into the lumen of the small intestine during the digestive process. Almost all of this water is also reabsorbed in the small intestine. Regardless of whether it is being secreted or absorbed, water flows across the mucosa in response to osmotic gradients. In the case of secretion, two distinct processes establish an osmotic gradient that pulls water into the lumen of the intestine: 1. Increases in luminal osmotic pressure resulting from influx and digestion of foodstuffs: The chyme that floods into the intestine from the stomach typically is not terribly hyperosmotic, but as its macromolecular components are digested, osmolarlity of that solution increases dramatically. Starch, for example, is a huge molecule that contributes only a small amount to osmotic pressure, but as it is digested, thousands of molecules of maltose are generated, each of which is as osmotically active as the original starch molecule. Thus, as digestion proceeds lumenal osmolarity increases dramatically and water is pulled into the lumen. Then, as the osmotically active molecules (maltose, glucose, amino acids) are absorbed, osmolarity of the intestinal contents decreases and water can be absorbed. 2. Crypt cells actively secrete electrolytes, leading to water secretion: The apical or lumenal membrane of crypt epithelial cells contain a ion channel of immense medical significance - a cyclic AMP-dependent chloride channel known also as the cystic fibrosis transmembrane conductance regulator or CFTR. Mutations in the gene for this ion channel result in the disease cystic fibrosis. This channel is responsible for secretion of water by the following steps: * Elevated intracellular concentrations of cAMP in crypt cells activate this channel, resulting in secretion of chloride ions into the lumen. * Accumulation of negatively-charged chloride anions in the crypt creates an electric potential that attracts sodium, pulling it into the lumen across the tight junctions - the net result is secretion of NaCl. * Secretion of NaCl into the crypt creates an osmotic gradient across the tight junction - water is drawn into the lumen. Abnormal activation of the cAMP-dependent chloride channel in crypt cells has resulted in the deaths of millions upon millions of people. Several types of bacteria produce toxins that strongly, often permanently, activate the adenylate cyclase in crypt enterocytes. This leads to elevated levels of cAMP, causing the chloride channels to essentially become stuck in the "open" position". The result is massive secretion of water that is manifest as severe diarrhea. Cholera toxin, produced by cholera bacteria, is the best known example of this phenomenon, but several other bacteria produce toxins that act similarly. Absorption in the Small Intestine: General Mechanisms -----------------------------------------------------------------------Virtually all nutrients from the diet are absorbed into blood across the mucosa of the small intestine. In addition, the intestine absorbs water and electrolytes, thus playing a critical role in maintenance of body water and acid-base balance. It's probably fair to say that the single most important process that takes place in the small gut to make such absorption possible is establishment of an electrochemical gradient of sodium across the epithelial cell boundary of the lumen. Don't turn off on me now - this is a critical concept and actually quite interesting! Also, as we will see, understanding this process has undeniably resulted in the saving of millions of lives. To remain viable, all cells are required to maintain a low intracellular concentration of sodium. In polarized epithelial cells like enterocytes, low intracellular sodium is maintained by a large number of Na+/K+ ATPases - so-called sodium pumps - embedded in the basolateral membrane. These pumps export 3 sodium ions from the cell in exchange for 2 potassium ions, thus establishing a gradient of both charge and sodium concentration across the basolateral membrane. In rats, as a model of all mammals, there are about 150,000 sodium pumps per small intestinal enterocyte which collectively allow each cell to transport about 4.5 billion sodium ions out of each cell per minute (J Membr Biol 53:119-128, 1980). Pretty impressive! This flow and accumulation of sodium is ultimately responsible for absorption of water, amino acids and carbohydrates. Aside from the electrochemical gradient of sodium just discussed, several other concepts are required to understand absorption in the small intestine. Also, dietary sources of protein, carbohydrate and fat must all undergo the final stages of chemical digestion just prior to absorption of, for example, amino acids, glucose and fatty acids. At this point, its easiest to talk separately about absorption of each of the major food groups, recognizing that all of these processes take place simultaneously. * * Water and electrolytes Carbohydrates, after digestion to monosaccharides * * Proteins, after digestion to small peptides and amino acids Neutral fat, after digestion to monoglyceride and free fatty acids Absorption of Water and Electrolytes -----------------------------------------------------------------------The small intestine must absorb massive quantities of water. A normal person or animal of similar size takes in roughly 1 to 2 liters of dietary fluid every day. On top of that, another 6 to 7 liters of fluid is received by the small intestine daily as secretions from salivary glands, stomach, pancreas, liver and the small intestine itself. By the time the ingesta enters the large intestine, approximately 80% of this fluid has been absorbed. Net movement of water across cell membranes always occurs by osmosis, and the fundamental concept needed to understand absorption in the small gut is that there is a tight coupling between water and solute absorption. Another way of saying this is that absorption of water is absolutely dependent on absorption of solutes, particularly sodium: * Sodium is absorbed into the cell by several mechanisms, but chief among them is by cotransport with glucose and amino acids - this means that efficient sodium absorption is dependent on absorption of these organic solutes. * Absorbed sodium is rapidly exported from the cell via sodium pumps - when a lot of sodium is entering the cell, a lot of sodium is pumped out of the cell, which establishes a high osmolarity in the small intercellular spaces between adjacent enterocytes. * Water diffuses in response to the osmotic gradient established by sodium - in this case into the intercellular space. It seems that the bulk of the water absorption is transcellular, but some also diffuses through the tight junctions. * Water, as well as sodium, then diffuses into capillary blood within the villus. Examine the animation above and consider the osmotic gradient between the lumen and the intercellular space (inside the villus). As sodium (green balls) is rapidly pumped out of the cell, it achieves very high concentration in the narrow space between enterocytes. The osmotic gradient is thus formed across apical cell membranes and their connecting junctional complexes. The arrow that appears denotes movement of water across the epithelium. Water is thus absorbed into the intercellular space by diffusion down an osmotic gradient. However, looking at the process as a whole, transport of water from lumen to blood is often against an osmotic gradient - this is important because it means that the intestine can absorb water into blood even when the osmolarity in the lumen is higher than osmolarity of blood. This ability is best explained by the "three compartment model" for absorption of water and, like many aspects of gut permeability, varies along the length of the gut. The proximal small intestine functions as a highly permeable mixing segment, and absorption of water is basically isotonic. That is, water is not absorbed until the ingesta has been diluted out to just above the osmolarity of blood. The ileum and especially the colon are able to absorb water against an osmotic gradient of several hundred milliosmols. Absorption of Monosaccharides -----------------------------------------------------------------------Simple sugars are far and away the predominant carbohydrate absorbed in the digestive tract, and in many animals the most important source of energy. Monosaccharides, however, are only rarely found in normal diets. Rather, they are derived by enzymatic digestion of more complex carbohydrates within the digestive tube. Particularly important dietary carbohydrates include starch and disaccharides such as lactose and sucrose. None of these molecules can be absorbed for the simple reason that they cannot cross cell membranes unaided and, unlike the situation for monosaccharides, there are no transporters to carry them across. This section will focus on understanding the processes involved in assimilation of three important carbohydrates: starch, lactose and sucrose. The key concepts involved in all three cases are that: * the final enzymatic digestion that liberates monosaccharides is conducted by enzymes that are tethered in the lumenal plasma membrane of absorptive enterocytes (socalled "brush border hydrolyases"). * glucose generated by digestion of starch or lactose is absorbed in the small intestine only by cotransport with sodium, a fact that has exceptionally important implications in medicine. Brush Border Hydrolases Generate Monosaccharides Polysaccharides and disaccharides must be digested to monosaccharides prior to absorption and the key players in these processes are the brush border hydrolases, which include maltase, lactase and sucrase. Dietary lactose and sucrose are "ready" for digestion by their respective brush border enzymes. Starch, as discussed previously, is first digested to maltose by amylase in pancreatic secretions and, in some species, saliva. Dietary lactose and sucrose, and maltose derived from digestion of starch, diffuse in the small intestinal lumen and come in contact with the surface of absorptive epithelial cells covering the villi where they engage with brush border hydrolases: * * * maltase cleaves maltose into two molecules of glucose lactase cleaves lactose into a glucose and a galactose sucrase cleaves sucrose into a glucose and a fructose At long last, we're ready to actually absorb these monosaccharides. Glucose, galactose and fructose are each taken into the enterocyte by facilitated diffusion. Glucose and galactose utilize the same transporter, while the fructose transporter is a separate entity. Absorption of Glucose: Transport Across the Intestinal Epithelium Absorption of glucose, or any molecule for that matter, entails transport from the intestinal lumen, across the epithelium and into blood. The transporter that carries glucose and galactose into the enterocyte is the sodium-dependent hexose transporter, known more formally as SGLUT-1. As the name indicates, this molecule transports both glucose and sodium into the cell and in fact, will not transport either alone. The essence of transport by the sodium-dependent hexose transporter involves a series of conformational changes induced by binding and release of sodium and glucose, and can be summarized as follows: * the transporter is initially oriented facing into the lumen - at this point it is capable of binding sodium, but not glucose * sodium binds, inducing a conformational change that opens the glucose-binding pocket * glucose binds and the transporter reorients in the membrane such that the pockets holding sodium and glucose are moved inside the cell * sodium dissociates into the cytoplasm, causing glucose binding to destabilize * glucose dissociates into the cytoplasm and the unloaded transporter reorients back to its original, outward-facing position The animation seen below depicts digestion of maltose and entry of the resulting glucose, along with sodium, into the enterocyte. Despite the simplicity of the diagram, you should easily be able to identify the sodium-dependent hexose transporter and "watch" its conformational changes. Also, imagine the corresponding process involving lactose and sucrose assimilation. Once inside the enterocyte, glucose and sodium must be exported from the cell into blood. We've seen previously how sodium is rapidly shuttled out in exchange for potassium by the battery of sodium pumps on the basolateral membrane, and how that process that maintains the electrochemical gradient across the epithelium. The energy stored in this gradient is actually what is driving glucose entry through the sodium-dependent hexose transporter described above. Recall also how the massive transport of sodium out of the cell establishes the osmotic gradient responsible for absorption of water. Glucose is tranported out of the enterocyte through a different transporter (called GLUT2) in the basolateral membrane. Glucose then diffuses "down" its concentration gradient into capillary blood within the villus. Absorption of Amino Acids and Peptides -----------------------------------------------------------------------Dietary proteins are, with very few exceptions, not absorbed. Rather, they must be digested into amino acids or di- and tripeptides first. In previous sections, we've seen two sources secrete proteolytic enzymes into the lumen of the digestive tube: * the stomach secretes pepsinogen, which is converted to the active protease pepsin by the action of acid. * the pancreas secretes a group of potent proteases, chief among them trypsin, chymotrypsin and carboxypeptidases. Through the action of these gastric and pancreatic proteases, dietary proteins are hydrolyzed within the lumen of the small intestine predominantly into medium and small peptides (oligopeptides). The brush border of the small intestine is equipped with a family of peptidases. Like lactase and maltase, these peptidases are integral membrane proteins rather than soluble enzymes. They function to further the hydrolysis of lumenal peptides, converting them to free amino acids and very small peptides. These endproducts of digestion, formed on the surface of the enterocyte, are ready for absorption. Absorption of Amino Acids The mechanism by which amino acids are absorbed is conceptually identical to that of monosaccharides. The lumenal plasma membrane of the absorptive cell bears at least four sodium-dependent amino acid transporters - one each for acidic, basic, neutral and amino acids. These transporters bind amino acids only after binding sodium. The fully loaded transporter then undergoes a conformational change that dumps sodium and the amino acid into the cytoplasm, followed by its reorientation back to the original form. Thus, absorption of amino acids is also absolutely dependent on the electrochemical gradient of sodium across the epithelium. Further, absorption of amino acids, like that of monosaccharides, contributes to generating the osmotic gradient that drives water absorption. The basolateral membrane of the enterocyte contains additional transporters which export amino acids from the cell into blood. These are not dependent on sodium gradients. Absorption of Peptides There is virtually no absorption of peptides longer than three amino acids. However, it seems that there is abundant absorption of di- and tripeptides in the small intestine. These small peptides are absorbed without dependence on sodium, probably by a single transport molecule. Once inside the enterocyte, the vast bulk of di- and tripeptides are digested into amino acids by cytoplasmic peptidases and exported from the cell into blood. Only a very small number of these small peptides enter blood intact. Absorption of Intact Proteins As emphasized, absorption of intact proteins occurs only in a few circumstances. In the first place, very few proteins get through the gauntlet of soluble and membrane-bound proteases intact. Second, "normal" enterocytes do not have transporters to carry proteins across the plasma membrane and they certainly cannot permeate tight junctions. One important exception to these general statements is that for a very few days after birth, neonates have the ability to absorb intact proteins. This ability, which is rapidly lost, is of immense importance because it allows the newborn animal to acquire passive immunity by absorbing immunoglobulins in colostral milk. In constrast to humans and rodents, there is no significant transfer of antibodies across the placenta in many animals (cattle, sheep, horses and pigs to name a few), and the young are born without circulating antibodies. If fed colostrum during the first day or so after birth, they absorb large quantities of immunoglobulins and acquire a temporary immune system that provides protection until they generate their own immune responses. The small intestine rapidly loses the capacity to absorb intact proteins - a process called closure - and consequently, animals that do not receive colostrum within the first few days after birth will likely die due to opportunistic infections. Absorption of Lipids -----------------------------------------------------------------------The bulk of dietary lipid is neutral fat or triglyceride, composed of a glycerol backbone with each carbon linked to a fatty acid. Additionally, most foodstuffs contain phospholipids, sterols like cholesterol and many minor lipids, including fat-soluble vitamins. In order for the triglyceride to be absorbed, two processes must occur: * Large aggregates of dietary triglyceride, which are virtually insoluble in an aqueous environment, must be broken down physically and held in suspension - a process called emulsification. * Triglyceride molecules must be enzymatically digested to yield monoglyceride and fatty acids, both of which can efficiently diffuse into the enterocyte The key players in these two transformations are bile salts and pancreatic lipase, both of which are mixed with chyme and act in the lumen of the small intestine. Emulsification, Hydrolysis and Micelle Formation Bile salts play their first critical role in lipid assimilation by promoting emulsification. As derivatives of cholesterol, bile salts have both hydrophilic and hydrophobic domains (i.e. they are amphipathic). On exposure to a large aggregate of triglyceride, the hydrophobic portions of bile salts intercalate into the lipid, with the hydrophilic domains remaining at the surface. Such coating with bile salts aids in breakdown of large aggregates or droplets into smaller and smaller droplets. Hydrolysis of triglyceride into monoglyceride and free fatty acids is accomplished predominantly by pancreatic lipase. The activity of this enzyme is to clip the fatty acids at positions 1 and 3 of the triglyceride, leaving two free fatty acids and a 2-monoglyceride. Lipase is a water-soluble enzyme, and with a little imagination, it's easy to understand why emulsification is a necessary prelude to its efficient activity. Shortly after a meal, lipase is present within the small intestine in rather huge quantities, but can act only on the surface of triglyeride droplets. For a given volume of lipid, the smaller the droplet size, the greater the surface area, which means more lipase molecules can get to work. The drug orlistat (Xenical) that is promoted for treatment of obesity works by inhibiting pancreatic lipase, thereby reducing the digestion and absorption of fat in the small intestine. As monoglycerides and fatty acids are liberated through the action of lipase, they retain their association with bile salts and complex with other lipids to form structures called micelles. Micelles are essentially small aggregates of mixed lipids and bile salts suspended within the ingesta. As the ingesta is mixed, micelles bump into the brush border and the lipids, including monoglyceride and fatty acids, are absorbed. Absorption and Transport into Blood Lipids are absorbed by a mechanism distinctly different from what we've seen for monosaccharides and amino acids. The major products of lipid digestion - fatty acids and 2monoglycerides - enter the enterocyte by simple diffusion across the plasma membrane. A considerable fraction of the fatty acids also enter the enterocyte via a specific fatty acid transporter protein in the membrane. Once inside the enterocyte, fatty acids and monoglyceride are transported into the endoplasmic reticulum, where they are used to synthesize triglyeride! Beginning in the endoplasmic reticulum and continuing in the Golgi, triglyceride is packaged with cholesterol, lipoproteins and other lipids into particles called chylomicrons. Remember where this is occurring - in the absorptive enterocyte of the small intestine. Chylomicrons are extruded from the Golgi into exocytotic vesicles, which are transported to the basolateral aspect of the enterocyte. The vesicles fuse with the plasma membrane and undergo exocytosis, dumping the chylomicrons into the space outside the cells. Because chylomicrons are particles, virtually all steps in this pathway can be visualized using an electron microscope, as the montage of images to the right demonstrates. Transport of lipids into the circulation is also different from what occurs with sugars and amino acids. Instead of being absorbed directly into capillary blood, chylomicrons are transported first into the lymphatic vessel that penetrates into each villus. Chylomicronrich lymph then drains into the system lymphatic system, which rapidly flows into blood. Blood-borne chylomicrons are rapidly disassembled and their constitutent lipids utilized throughout the body. If you are interested in confirming for yourself at least some of the processes described above, you should perform the following experiment: * Consume a cup of rich cream or a sack of fast-food French fries. * Do something productive like studying for about 30 minutes. * Draw a blood sample from yourself (a capillary tube is enough) - use an anticoagulant to prevent clotting. * Centrifuge the blood sample to separate cells and plasma. When you examine your plasma it will look distinctly milky due to the presence of billions of light-reflecting chylomicrons (the condition is called lipemia). If you want extra credit, continue the blood sampling every 15 minutes until your plasma clears, then plot your results on graph paper. Absorption of Minerals and Metals -----------------------------------------------------------------------The vast bulk of mineral absorption occurs in the small intestine. The best-studied mechanisms of absorption are clearly for calcium and iron, deficiencies of which are significant health problems throughout the world. Minerals are clearly required for health, but most also are quite toxic when present at higher than normal concentrations. Thus, there is a physiologic challenge of supporting efficient but limited absorption. In many cases intestinal absorption is a key regulatory step in mineral homeostasis. Calcium The quantity of calcium absorbed in the intestine is controlled by how much calcium has been in the diet during recent periods of time. Calcium is absorbed by two distinct mechanims, and their relative magnitude of importance is set by dietary calcium "history": * Active, transcellular absorption occurs only in the duodenum when calcium intake has been low. This process involves import of calcium into the enterocyte, transport across the cell, and export into extracellular fluid and blood. Calcium enters the intestinal epithelial cells through voltage-insensitive channels and is pumped out of the cell via a calciumATPase. The rate limiting step in transcellular calcium absorption is transport across the epithelial cell, which is greatly enhanced by the carrier protein calbindin, the synthesis of which is totally dependent on vitamin D. * Passive, paracellular absorption occurs in the jejunum and ileum, and, to a much lesser extent, in the colon when dietary calcium levels have been moderate or high. In this case, ionized calcium diffuses through tight junctions into the basolateral spaces around enterocytes, and hence into blood. Such transport depends on having higher concentrations of free calcium in the intestinal lumen than in blood. Phosphorus Phosphorus is predominantly absorbed as inorganic phosphate in the upper small intestine. Phosphate is transported into the epithelial cells by contransport with sodium, and expression of this (or these) transporters is enhanced by vitamin D. Iron Iron homeostasis is regulated at the level of intestinal absorption, and it adequate but not excessive quantities of iron be absorbed from the absorption can lead to iron-deficiency disorders such as anemia. On excessive iron is toxic because mammals do not have a physiologic elimination. is important that diet. Inadequate the other hand, pathway for its Iron is absorbed by villus enterocytes in the proximal duodenum. Efficient absorption requires an acidic environment, and antacids or other conditions that interfere with gastric acid secretion can interfere with iron absorption. Ferric iron (Fe+++) in the duodenal lumen is reduced to its ferrous form through the action of a brush border ferrireductase. Iron is the cotransported with a proton into the enterocyte via the divalent metal transporter DMT-1. This transporter is not specific for iron, and also transports many divalent metal ions. Once inside the enterocyte, iron follows one of two major pathways. Which path is taken depends on a complex programming of the cell based on both dietary and systemic iron loads: * Iron abundance states: iron within the enterocyte is trapped by incorporation into ferritin and hence, not transported into blood. When the enterocyte dies and is shed, this iron is lost. * Iron limiting states: iron is exported out of the enterocyte via a transporter (ferroportin) located in the basolateral membrane. It then binds to the iron-carrier transferrin for transport throughout the body. Copper There appear to be two processes responsible for copper absorption - a rapid, low capacity system and a slower, high capacity system, which may be similar to the two processes seen with calcium absorption. Many of the molecular details of copper absorption remain to be elucidated. Inactivating mutations in the gene encoding an intracellular copper ATPase have been shown responsible for the failure of intestinal copper absorption in Menkes disease. A number of dietary factors have been shown to influence copper absorption. For example, excessive dietary intake of either zinc or molybdenum can induce secondary copper deficiency states. Zinc Zinc homeostasis is largely regulated by its uptake and loss through the small intestine. Although a number of zinc transporters and binding proteins have been identified in villus epithelial cells, a detailed picture of the molecules involved in zinc absorption is not yet in hand. Intestinal excretion of zinc occurs via shedding of epithelial cells and in pancreatic and biliary secretions. A number of nutritional factors have been identified that modulate zinc absorption. Certain animal proteins in the diet enhance zinc absorption. Phytates from dietary plant material (including cereal grains, corn, rice) chelate zinc and inhibit its absorption. Subsistance on phytate-rich diets is thought responsible for a considerable fraction of human zinc deficiencies. Ontogeny of Small Intestinal Function -----------------------------------------------------------------------Survival depends on the ability of neonates to absorb nutrients beginning immediately after birth, and the intestinal mucosa is certainly functional at that time. However, the neonate's mucosa is 'fine-tuned' to exploit it's standard diet - milk - and changes in distinctive ways over time to become nearly adult-like at the time of weaning. Mice, humans and pigs have been the most studied with regard to ontogeny of mucosal function. Changes have been characterized for both digestive and absorptive processes. Carbohydrate Digestion and Absorption A well studied example of ontogenic change is in small intestinal disaccharidase activity. Lactose is the major, almost sole dietary carbohydrate for neonates, but, except for some humans, is not consumed by adult animals. On the other hand, neonates do not normally ingest sucrose or starch, but adults often do. Neonates and adults alike are unable to absorb dietary disaccharides and require brush border disaccharidases to hydrolyze those molecules into absorbable monosaccharides. Considering these facts, it would make physiologic sense for neonatal intestine to have high lactase activity, but low sucrase and maltase activities, and for lactase to diminish dramatically and sucrase/maltase to increase by the time of weaning. Indeed, this is exactly what happens in rodents, pigs and, for the most part, humans. In both pigs and humans, lactase activity is first observed early in gestation (e.g. 8-9 weeks in human gestation) and rises rather dramatically during the third trimester to achieve high levels during the first week of life. The top figure to the right depicts brush border lactase concentrations in different regions of the neonatal pig intestine, from one through six weeks of age. Note the steady decline in lactase concentration throughout the small intestine, a pattern seen in essentially all animals studied. The bottom figure to the right shows the inverse relationship between lactase and sucrase concentrations in the small intestine of young rats. In humans, the change in sucrase expression is less dramatic, and sucrase concentrations are relatively stable from birth through adulthood. The mechanisms responsible for these developmental changes in intestinal disaccharidase expression remain poorly defined. Changes in diet clearly play some role, but do not appear to be the major regulator. For example, prolonging the period of suckling in rats causes a delay in decline of lactase expression, but does not alter the timing of increased sucrase expression. A postnatal rise in secretion of glucocorticoids also seems to modulate expression of disaccharidase genes, but again, is clearly not the major controller. Treating young rats with the glucocorticoid corticosterone slightly accelerated the rise in sucrase expression, but did not affect lactase expression. Hexoses are absorbed by the same transport mechanisms in neonates and adults and there are not major quantitative changes in capacity for transport. For example, in both neonates and adults glucose is transported from the lumen via the sodium-dependent hexose transporter. Expression of this protein does not undergo significant changes during postnatal development in many animals, but in ruminants, there is a dramatic decrease in expression of this transporter (SGLUT1) during the first few weeks after birth, and very low levels of expression after weaning. As might be expected from changes in sucrase expression, fructose transport is low during the suckling period and rises significantly at weaning. Protein Digestion and Absorption Mechanisms of protein digestion and absorption are distinctly different between neonates and adults. Brush border peptidases appear to be present in the neonatal mucosa, but their expression does increase substantially at weaning. Also, amino acid transporters are functional at birth, although there may be quantitative changes as the animal matures. Most dramatically however, the neonatal intestine efficiently transports intact proteins across the mucosa for varying periods of time after birth. In rodents, there is minimal secretion of gastric and pancreatic proteases in the neonatal period. Mucosal epithelial cells pinocytose intact proteins and degrade them in lysosomes. One important exception to this pathway is seen in absorption of immunglobulins, which bind to Fc receptors and are transcytosed across the epithelium instead of being targeted to lysosomes. Neonatal pigs seem to use a slightly different strategy - their proteolytic systems are more intact, but intestinal contents are rich in protease inhibitors (presumably from serum), which also allow intact proteins to reach the epithelium for pinocytosis. Finally human neonates also absorb macromolecules intact and this ability persists well into childhood, but the mechanisms allowing it is not well studied. Other Developmental Changes In neonatal rodents, calcium is absorbed by a nonsaturable, vitamin D-independent process. By the end of weaning, calcium transport is adult-like, in that it saturable, dependent on vitamin D and concentrated in the duodenum. The Three Compartment Model for Transport of Water Across Epithelium -----------------------------------------------------------------------An early observation by physiologists studying absorption of water in the gut was that fluid absorption did not cease when the the lumenal contents were hyperosmolar. They correctly deduced that the intestinal epithelium can transport water against an osmotic gradient, but were unable to explain how this occurred. There is still considerable uncertainty about how water is absorbed in such a situation, but Curran and Macintosh presented a explanation known the "three compartment - two membrane model" that accounts for many aspects of water transport in the gut and other tissues. It is proposed that the epithelium consists of three compartments separated by two membranes which differ in permeability, as shown diagramatically below: In this situation, water will move against an osmotic gradient from compartment A to compartment C as long as two conditions are met: * * The osmolarity in compartment B is greater than in compartment A. The permeability of membrane I is less than that of membrane II. In such a case, the higher osmolarity in compartment B relative to A or C provides the driving force for movement of water from A to B. As water flows into compartment B, the hydrostatic pressure in that compartment increases, forcing water to flow through membrane B and into the lowest osmolarity compartment C. The anatomic correlates to the two membranes in this model are not known, but membrane I (least permeable) may be the basolateral membrane of the enterocyte and membrane II (most permiable) could be the basement membrane and/or capillary endothelial cell. The Large Intestine: Introduction and Index ------------------------------------------------------------------------ The large intestine is the last attraction in digestive tube and the location of the terminal phases of digestion. In comparison to other regions of the tube, there are huge differences among species in the relative size and complexity of the large intestine. Nonetheless, in all species it functions in three processes: * Recovery of water and electrolytes from ingesta: By the time ingesta reaches the terminal ileum, roughly 90% of its water has been absorbed, but considerable water and electrolytes like sodium and chloride remain and must be recovered by absorption in the large gut. * Formation and storage of feces: As ingesta is moved through the large intestine, it is dehydrated, mixed with bacteria and mucus, and formed into feces. The craftsmanship (for want of a better term) with which this is carried out varies among species. * Microbial fermentation: The large intestine of all species teems with microbial life. Those microbes produce enzymes capable of digesting many of molecules that to vertebrates are indigestible, cellulose being a premier example. The extent and benefit of fermentation also varies greatly among species. Core concepts in physiology of the large intestine are presented as the following topics: * * * * Gross and microscopic anatomy of the large intestine Absorption and secretion, and formation of feces in the large intestine Large intestinal motility Microbial fermentation Advanced and supplemental topics related to physiology of the large intestine: * Intestinal gas production Falta intestino grueso todo Microbial Fermentation -----------------------------------------------------------------------Fermentation is the enzymatic decomposition and utililization of foodstuffs, particularly carbohydrates, by microbes. Fermentation takes place in the large bowel of all animals, but there are major differences in its contribution to the nutrition of different species. In carnivores like dogs and cats, and even in omnivores like humans, fermentation generates very few calories. In herbivores, however, fermentation is a way of life. The large intestine does not produce its own digestive enzymes, but contains huge numbers of bacteria which have the enzymes to digest and utilize many substrates. In all animals, two processes are attributed to the microbial flora of the large intestine: * * Digestion of carbohydrates not digested in the small intestine. Synthesis of vitamin K and certain B vitamins. Cellulose is common constituent in the diet of many animals, including man, but no mammalian cell is known to produce a cellulase. Several species of bacteria in the large bowel synthesize cellulases and digest cellulose. Importantly, the major end products of microbial digestion of cellulose and other carbohydrates are volatile fatty acids, lactic acid, methane, hydrogen and carbon dioxide. Fermentation is thus the major source of intestinal gas. Volatile fatty acids (acetic, proprionic and butyric acids) generated from fermentation can be absorbed by diffusion in the colon. You obtain a few calories from eating a salad. A rabbit, on the other hand, has a relatively huge fermentation vat (cecum), and obtains much of its energy from the plants it consumes. Synthesis of vitamin K by colonic bacteria provides a valuable supplement to dietary sources and makes clinical vitamin K deficiency rare. Similarly, formation of B vitamins by the microbial flora in the large intestine is useful to many animals. They are not absorbed in the large intestine, but are present in feces. The behavior of coprophagy or eating feces seen particularly in rodents, rabbits and other animals is thought to be a behavioral adaption to recovery of these valuable resources. A more comprehensive description of fermentation is presented in the section on digestive physiology of herbivores. Gross and Microscopic Anatomy of the Large Intestine -----------------------------------------------------------------------The large intestine is that part of the digestive tube between the terminal ileum and anus. Depending on the species, ingesta from the small intestine enters the large intestine through either the ileocecal or ileocolic valve. Within the large intestine, three major segments are recognized: * the cecum is a blind-ended pouch that in humans carries a worm-like extension called the vermiform appendix. * the colon constitutes the majority of the length of the large intestine and is subclassified into ascending, transverse and descending segments. * the rectum is the short, terminal segment of the digestive tube, continuous with the anal canal. The variation in relative dimension of the large intestine is largely correlated with diet. In herbivores like horses and rabbits which depend largely on microbial fermentation, the large intestine is very large and complex. Omnivores like pigs and humans have a substantial large intestine, but nothing like that seen in herbivores. Finally, carnivores such as dogs and cats have a simple and small large intestine. There are many similarities in the histologic structure of the mucosa in large and small intestine. The most obvious difference is that the mucosa of the large intestine is devoid of villi. It has numerous crypts which extend deeply and open onto a flat lumenal surface. The stem cells which support rapid and continuous renewal of the epithelium are located either at the bottom or midway down the crypts. These cells divide to populate the cryptal and surface epithelium. Mucus-secreting goblet cells are also much more abundant in the colonic epithelium than in the small gut. The image above shows a section of colon from a dog. Note the crypts extending from the lumen, and the numerous, foamy goblet cells that populate the epithelium of the crypts. Picture 25a Absorption, Secretion and Formation of Feces in the Large Intestine -----------------------------------------------------------------------US Forest Service Travel Warning The last two years have seen unprecedented growth in bear populations in the Rocky Mountain region. As Spring approaches, tourists are advised to wear small bells attached to their clothing, as this will frighten away most bears. Tourists are also cautioned to watch the ground on the trail for bear droppings. Be particularly alert for the presence of Grizzly bear droppings, which are easily recognized because they usually contain small bells. To a first approximation, absorption and secretion in the colon is straighforward: * * Absorption: water, sodium ions and chloride ions Secretion: bicarbonate ions and mucus Water, as always, is absorbed in response to an osmotic gradient. The mechanism responsible for generating this osmotic pressure is essentially identical to what was seen in the small intestine - sodium ions are transported from the lumen across the epithelium by virtue of the epithelial cells having very active sodium pumps on their basolateral membranes and a means of absorbing sodium through their lumenal membranes. The colonic epithelium is actually more efficient at absorbing water than the small intestine and sodium absorption in the colon is enhanced by the hormone aldosterone. Chloride is absorbed by exchange with bicarbonate. The resulting secretion of bicarbonate ions into the lumen aids in neutralization of the acids generated by microbial fermentation in the large gut. Goblet cells are abundant in the colonic epithelium, and secrete mucus in response to tactile stimuli from lumenal contents, as well as parasympathetic stimuli from pelvic nerves. Mucus is an important lubricant that protects the epithelium, and also serves to bind the dehydrated ingesta to form feces. Normal feces are roughly 75% water and 25% solids. The bulk of fecal solids are bacteria and undigested organic matter and fiber. The characteristic brown color of feces are due to stercobilin and urobinin, both of which are produced by bacterial degradation of bilirubin. Fecal odor results from gases produced by bacterial metabolism, including skatole, mercaptans, and hydrogen sulfide. Large Intestinal Motility -----------------------------------------------------------------------Three patterns of motility are observed the colon: * Segmentation contractions which chop and mix the ingesta, presenting it to the mucosa where absorption occurs. These contractions are quite prominent in some species, forming sacculations in the colon known as hausta. * Antiperistaltic contractions propagate toward the ileum, which serves to retard the movement of ingesta through the colon, allowing additional opportunity for absorption of water and electrolytes. Peristaltic contractions, in addition to influx from the small intestine, facilitate movement of ingesta through the colon. * Mass movements constitute a type of motility not seen elsewhere in the digestive tube. Known also as giant migrating contractions, this pattern of motility is like a very intense and prolonged peristaltic contraction which strips an area of large intestine clear of contents. In periods between meals, the colon is generally quiescent. Following a meal, colonic motility increases significantly, due to signals propagated through the enteric nervous system - the so called gastrocolic and duodenocolic reflexes, manifestation of enteric nervous system control. In humans, the signal seems to be stimulated almost exclusively by the presence of fat in the proximal small intestine (well, some people find this interesting). Additionally, distension of the colon is a primary stimulator of contractions. Several times each day, mass movements push feces into the rectum, which is usually empty. The gastrocolic reflex mentioned above is a stimulus for this. Distension of the rectum stimulates the defecation reflex. This is largely a spinal reflex mediated via the pelvic nerves, and results in reflex relaxation of the internal anal sphincter followed by voluntary relaxation of the external anal sphincter and defecation. In humans and "housetrained" animals, defecation can be prevented by voluntary constriction of the external sphincter. When this happens, the rectum soon relaxes and the internal sphincter again contracts, a state which persists until another bolus of feces is forced into the rectum.