notes: periodic table development
advertisement

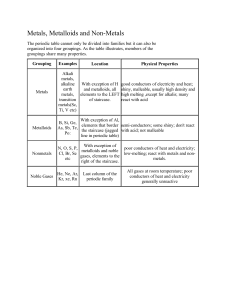
NOTES: PERIODIC TABLE DEVELOPMENT Objective: Describe the history and development of the periodic table and identify the current method of organization Come up with a list of 20 food items… If you were making a chart where each of these food items is logically organized, how would you build this chart? Build an example chart… Chemists did the same thing with the elements that had been discovered. Chemists wanted to organize a chart that displayed the elements in a logical way. By 1870, we had discovered about 70 elements, and chemists were overwhelmed with learning the properties of so many new elements. They wanted to organize the elements in a way that would help them remember the properties of elements. I. JOHN NEWLANDS - Arranged the elements by _______________________________ - Properties of elements repeat every eighth element (elements 1 and 9 would have similar properties) - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 - Called ______________ - Periodic: II. MENDELEEV - Like Newlands, arranged by atomic mass - Put elements with similar properties into columns together - Arranged a periodic table: - Left spaces in table for _________________________ Correctly predicted ___________________________ Widely accepted by scientists for many years III. TODAY’S PERIODIC TABLE: Moseley - Mendeleev’s table was not completely correct - What happened to improve Mendeleev’s model? - NEW table: arranged by INCREASING ______________ What is the atomic number equal to in the periodic table? - PERIODIC LAW: - Each vertical GROUP of the periodic table shares similar chemical properties List three elements that share chemical properties with each of the following elements: 1. Barium 2. Bromine 3. Gold How are the electron configurations for elements in a group similar? Homework for section 6-1 PART 1: Come up with four potential quiz questions covering the material we went over in class today. You must use at least two types of questions. Your options are multiple choice, open response, or fill in the blank. Provide the answer to each question. The Modern Periodic Table Objective: Label important groups on the periodic table and identify the position of various categories of elements Horizontal Rows: PERIODS - Total of ___________ periods Columns: GROUPS - There is a total of _________ groups - Two methods of labeling groups: - 1. - 2. Representative Elements: - Groups __________________________ - Why are they called representative elements? Transition Elements: - Groups ______________________ - All metals Metals: - On ___________side of staircase in periodic table - Properties: 1. 2. 3. 4. 5. Groups of Metals: - Alkali Metals: ________________ - Hydrogen is the exception (NOT a metal) - Properties: - 1. - 2. - 3. - Alkaline Earth Metals: _______________ - Properties - 1. - 2. - Inner Transition Metals: ___________________________ - Called the Lanthanide (1st period) and Actinide (2nd period) series Nonmetals: - Located to the ______________ of the staircase Properties: 1. 2. Halogens: ___________ Properties: 1. 2. Noble Gases: _______________ Properties: Metalloids: - Located _________ the staircase Have properties of ______________________ PRACTICE PROBLEMS: 1. Identify the following elements as transition metals, inner transition metals, or representative elements. a. Copper b. Ac c. Mercury d. Br e. Rubidium f. Promethium (Pm) 2. Identify the following elements as metals, nonmetals, or metalloids a. Chlorine b. Sodium c. Calcium d. Xenon e. Antimony f. Germanium g. Promethium h. Carbon i. Sulfur 3. Identify the group name of the following elements (i.e. alkali metals, alkaline earth metals, halogens, noble gas) a. Ar b. Rb c. Mg d. Ba e. I f. F Section 6.1: Homework 1. Compare and contrast the characteristics of metals, nonmetals, and metalloids: Metals Nonmetals Metalloids 2. What types of elements does the “staircase” on the periodic table separate? 3. Describe the properties of the alkali earth metals: 4. Describe the properties of the halogens: 5. Describe the properties of the noble gases: 6. On the attached periodic table… 1. Identify the REPRESENTATIVE elements by CIRCLING their group numbers 2. Identify the INNER TRANSITION METALS with an arrow pointing to their periods 3. Identify the TRANSITION METALS with an arrow pointing to their groups 4. HIGHLIGHT the staircase with your pen or a different color 5. COLOR the metals one color, the nonmetals another color, and the metalloids a third color. Be sure to include a key so I know which color corresponds to which category. 6. LABEL the alkali metals, the alkaline earth metals, the halogens, and the noble gases 7. Circle the elements that are liquids at room temperature