isotopes, relative atomic mass and mass spectrometry
advertisement

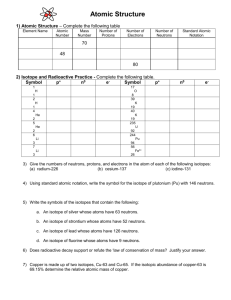
ISOTOPES, RELATIVE ISOTOPIC MASS, MASS SPECTROMETRY AND RELATIVE ATOMIC MASS ISOTOPES - DIFFERENT FORMS OF AN ELEMENT Atoms within an element that have the same number of protons and electrons but a different number of neutrons are called ISOTOPES of that element. Isotopes of an element are chemically identical because it is the number of protons and electrons, rather than the number of neutrons, which determines the characteristic chemical behavior of an element. Different isotopes of the same element are referred to by their mass numbers, eg. the most common isotopes of carbon are referred to as carbon - 12 , carbon -13 and carbon -14 Most elements have two or more naturally occurring isotopes. The element zinc, for example, may contain five isotopes, as shown in below: Isotope Name Isotope Symbol Natural % abundance 64 Zinc 64 Zn 48.89 Zn 27.81 Zn 4.11 Zn 18.57 Zn 0.62 30 66 Zinc 66 30 67 Zinc 67 30 68 Zinc 68 30 70 Zinc 70 30 Although all zinc atoms contain 30 protons and 30 electrons, each isotope contains a different number of neutrons. Zinc - 64 contains neutrons and zinc-70 contains neutrons. Isotopes of an element have slightly different masses. Is this a difference in a physical or chemical property? RELATIVE ISOTOPIC MASS It might seem that we could determine the mass of an atom of a particular atom by adding together the masses of the protons, neutrons and electrons that make it up, but atoms are too small to be weighed directly, (although their masses can be determined indirectly by measuring their effect on each other). Atoms are so tiny that knowing the mass of any individual atom isn’t very useful, as chemists never carry out any experimental or practical work on just one atom at a time. To deal with this, chemists invented a relative scale of atomic masses. A relative scale is one in which all measurements are compared to one standard or reference measure. For example, if a student of Year 11 chemistry is 15 years of age, has a father of age 45 and a younger sister who is 9 years old, he could invent a relative scale of ages for these members of his family. According to this relative scale, he would be the reference and so if he was given an arbitrary value of 1 unit, his father would be 3 units (as he is 45/15 or three times older than the reference person) and the younger sister would be 0.6 units (as she is 9/15 the age of the reference). [Note that relative scales have no units, because they are just comparisons of one quantity to another.] What would be the relative scale age of the student’s mother if she was 40 years of age? Another example: The three-door Suzuki Swift has a curb weight of 745 kg, compared to a Holden Commodore which weighs 1600 kg. A typical 18-wheel “B double” semi-trailer, when fully laden, can weigh up to 79 tonnes (1 tonne = 1000 kg). If the Commodore has a relative mass of one unit, what is the mass of the Suzuki and the semi on the same relative scale? From this example you can see that the advantage of relative scales is that very large or very small numbers can compared relatively easily. To make a relative scale of atomic masses, chemists chose the most abundant isotope of the element carbon, the carbon-12 isotope, as the reference. This carbon–12 isotope was given a relative mass of exactly 12 units, not 1 unit. Page 2 of 8 The element carbon was chosen as the reference for a number of important reasons: • carbon is very cheap and is widely available • it is relatively easy to isolate and purify this isotope • carbon is not toxic in any way. The relative isotopic mass (RIM or Ir ) of an isotope is the mass of an atom of that isotope relative to the mass of an atom of 12C taken as 12 exactly. Examples: An atom of an isotope of magnesium is twice as heavy as an atom of What is the RIM of this isotope? RIM =12 x 2 = 24 An atom of an isotope of hydrogen has ¼ the mass of an atom of What is the RIM of this isotope? 12 12 C. C RIM =12 x ¼ = 3 3 What is the likely nucleide symbol for this isotope? An isotope of silver has an RIM of 108. How much heavier is it than an atom of 12 H C? An isotope has an RIM of 16 (i) How much heavier is it than an atom of 12 C? (ii) Which of the following nucleide symbols is most likely to represent this isotope? A. 32 S 16 B. 16 N 7 C. 16 Cl 17 D. 33 S 16 DETERMINING THE RELATIVE ISOTOPIC MASS OF AN ELEMENT Page 3 of 8 A mass spectrometer may be used to determine the relative isotopic mass of a particular atom. A mass spectrometer works in five main stages to record the mass and relative number of the ions produced by a sample of an element. [ go to http://www.colby.edu/chemistry/OChem/DEMOS/MassSpec.html for a great animation showing how a mass spec. works] 1. The test sample is heated until it vaporizes (forms a gas) 2. The gas is bombarded with high energy electrons that “knock off” at least one valence electron from some of the gas atoms, forming positively charged ions. (This even happens to elements that would normally be expected to form negative ions (eg. chlorine) or never form ions at all (eg. argon)) 3. The positive ions are accelerated by an electric field so that they move rapidly through the machine. 4. The rapidly moving positively charged ions behave like “mini magnets” and can be deflected (“pushed off course”) by the magnetic field of a strong electromagnet. The inside of the mass spectrometer is “evacuated” (all the air and any other gases are removed before the gas sample enters the machine.) This allows the ions to move through the machine without hitting any random gas molecules from the air. (If ions are travelling at different speeds due to collisions with air molecules, the amount they are deflected by the magnetic field will not be the same each time.) Lighter ions are deflected more than heavier ones. 5. As they pass out of the magnetic field, ions are detected by an ion detector which Page 4 of 8 records the position of the ions on the screen and the number of ions that hit the screen at each position. These two pieces of information are used to produce a mass spectrum for the sample. Different elements, when analysed by mass spectrometry, produce different spectra: the mass spectrum of an element is like a ‘fingerprint’ of the element. [In VCE unit 3, the use of the mass spectrometer to identify both compounds and elements is explained.] Simple Mass Spectra The simplest mass spectra belong to elements that are monatomic (exist as single atoms), eg: analysing the mass spectrum for boron (B) The number of peaks tells us how many isotopes the element has. The two peaks in the mass spectrum of boron indicates that there are 2 isotopes of boron The position of each peak on the horizontal axis (“x” –axis) indicates the relative isotopic mass. The isotopes have RIM’s of 10 and 11 on the 12C scale. The relative heights of the peaks correspond to the relative abundance of the isotopes. The peak for 11B is 100 and the peak for 10B is 25, so there is four times as much 11B as 10B. Expressed as a percentage relative abundance 11B is 80% and 10B is 20%. Example Page 5 of 8 Above is an imaginary mass spectrometer ‘trace’ of an element X. How many isotopes does element X have? What is the RIM of each isotope? What is the % relative abundance of each isotope? RELATIVE ATOMIC MASS Relative atomic mass (RAM or Ar) of an element is defined as: the weighted average of the RIM’s of the isotopes of the element relative to the 12C atom, taken as 12 exactly. Most elements occur in nature as a mixture of isotopes. For example, “natural” chlorine is a gas containing approximately 75.80% of the isotope 35Cl (RIM = 34.969) and 24.20% of the isotope 37Cl (RIM = 36.966). In any sample of “natural” chlorine there will be more of the isotope 35Cl than of the isotope 37Cl, so the relative atomic mass of chlorine will be will be closer to 35 than to 37; so the average relative mass of one chlorine atom must take into account the relative abundance of each isotope as well as its isotopic mass and to calculate the RAM of chlorine: = = (relative isotopic mass x % abundance) + (relative isotopic mass x % abundance) 34.969 x 75.80 + 100 36.966 x 24.20 = 2650.65 + 100 = 100 35.45 (notice answer can only be quoted to 4 sig.figs) Problems: Page 6 of 8 894.58 = 35.452 1. A sample of an element consists of three isotopes. (i) makes up 90.5% of the element and has RIM of 19.92; (ii) makes up 0.279% of the element and has RIM of 20.994; (iii) makes up 9.22% of the element and has RIM of 21.990. Determine the relative atomic mass of the element, then use the RAM/Ar data in the periodic table to identify the element. 2. Using the mass spectrum of magnesium provided above, answer the following questions. (a) How many isotopes are present? (b) What is the RIM of each isotope? (c) Show the nucleide symbols for each isotope. (d) Calculate the relative atomic mass (RAM) of magnesium. 3. The RAM listed in the Periodic Table for Magnesium is 24.305. A year 11 student claims “there isn’t a single magnesium atom in the universe with a mass of 24.305.” Is this statement correct? Explain your answer. MULTIPLE CHOICE QUESTIONS (Choose the best alternative) Page 7 of 8 1. Carbon is considered to be an element because A. all of its atoms have the same atomic number B. one of its isotopes has mass number 12 C. it is a substance present in all living things D. it has the same number of neutrons as protons in its nucleus 2. An atom of the isotope 39K contains: A. 19 protons, 19 neutrons, 20 electrons B. 19 protons, 39 neutrons, 19 electrons C. 19 protons, 20 neutrons, 19 electrons D. 20 protons, 19 neutrons, 20 electrons 3. The mass spectrum of an element is shown below A. three isotopes are shown with masses in the ratio 206:207:208 and relative abundances 1%, 1% and 2% B. the three isotopes have the same mass number but different atomic numbers C. isotope 206 and 207 have relative % abundances of 25% D. the relative atomic mass of this element is closer to 208 than to 207 4. The element gallium, symbol Ga, consists of 60% by mass of and 40% of 71Ga. Its relative atomic mass is represented by A (69 + 71) / 2 B. (60 + 69 + 40 + 71) /4 C ( 60 x 69) + (40 x 71) / (69 + 71) D. ( [60 x 69] + [40 x 71]) /100 Page 8 of 8 69 Ga