Sulfoxidation
advertisement

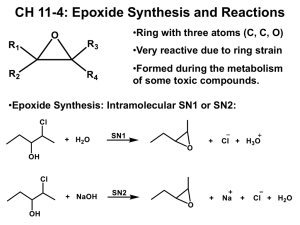
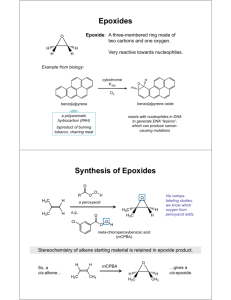
1 Sulfoxidation n a phase-II detoxification pathway that occurs in the liver and in which sulfite oxidase converts sulfites, such as those found in food preservatives and colorings, garlic compounds, and chlorpromazine into sulfates so they can be safely eliminated from the body. Sulfoxidation is another reaction catalyzed by POMs (Poly OxoMetalates). This catalysis is applicable not only to the production of sulfoxides, which are generally more expensive than the corresponding sulfones, there is also a large interest in their use as detoxifying agents. Mustard gas, dichloroethyl sulfide, is catalytically oxidized by some POMs to form the much less dangerous sulfoxide. These catalysts must proceed with virtually complete selectivity for the sulfoxide, because over oxidation can lead to the sulfone, which is more toxic than the sulfide itself. There is current research underway to attach these POMs to support structures such as silica or cotton . These types of materials could then be placed in soldiers’ uniforms to help protect them from mustard gas exposure. Sulfides also exist as atmospheric pollutants, so there is also a civilian interest in these materials as air purifiers. n-oxidation An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O− (sometimes written as R3N=O or R3N→O). In the strict sense the term amine oxide applies only to oxides of tertiary amines including nitrogen-containing aromatic compounds like pyridine, but is sometimes also used for the analogous derivatives of primary and secondary amines. Amine oxides are used as protecting group for amines and as chemical intermediates. Long-chain alkyl amine oxides are used as nonionic surfactants and foam stabilizers. Amine oxide are highly polar molecules have a high polarity close to that of quaternary ammonium salts. Small amine oxides are very hydrophilic and have an excellent water solubility and a very poor solubility in most organic solvents. Amine oxides are weak bases with a pKa of around 4.5 that form R3N+-OH, cationic hydroxylamines, upon protonation at a pH below their pKa. Pyridine N-oxide is a crystalline solid with melting point 62-67°C and soluble in water NMethylmorpholine N-oxide is an oxidant. Epoxidation An epoxide is a cyclic ether with only three ring atoms. This ring approximately is an equilateral triangle (i.e., its bond angles are about 60°) which makes it highly strained. The strained ring makes epoxides more reactive than other ethers, especially towards nucleophiles. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in chloromethyloxirane. As a functional group epoxides obtain the epoxy prefix such as in the compound 1,2-epoxycycloheptane which can also be called cycloheptene epoxide. 2 A polymer containing unreacted epoxide units is called a polyepoxide or an epoxy. Epoxy resins are used as adhesives and structural materials. Polymerization of an epoxide gives a polyether, for example ethylene oxide polymerizes to give polyethylene glycol, also known as polyethylene oxide. Epoxides are usually created by one of the following reactions: Olefin peroxidation Olefin peroxidation, also known as the Prilezhaev reaction involves the oxidation of an alkene with a peroxide, usually a peroxyacid like m-CPBA or with a dioxirane like DMDO. An example is the epoxidation of styrene with perbenzoic acid to styrene oxide. The reaction proceeds via what is commonly known as the Butterfly Mechanism.It is easiest to consider the oxygen to be an electrophile, and the alkene a nucleophile, although they both operate in that capacity, and the reaction is considered to be concerted (the numbers in the mechanism below are for simplification). Related processes include some catalytic enantioselective reactions, such as the: Sharpless epoxidation Jacobsen epoxidation Shi epoxidation Intramolecular SN2 substitution This method is a variant of the Williamson ether synthesis. In this case, the alkoxide ion and the halide are right next to each other in the same molecule (such compounds are generically called halohydrins), which makes this a simple ring closure reaction. For example, with 2-chloropropanol. Johnson-Corey-Chaykovsky reaction In the Johnson-Corey-Chaykovsky reaction epoxides are generated from carbonyl groups and sulfonium ylides. Reactions Typical epoxide reactions are listed below. Nucleophilic addition to an epoxide can be base or acid catalyzed. 3 Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocation, i.e. the most substituted carbon (similar to a halonium ion). Under basic conditions, the nucleophile attacks the least substituted carbon, in accordance with standard SN2 nuclephilic addition reaction process. Hydrolysis of an epoxide in presence of an acid catalyst generates a glycol. The hydrolysis process of epoxides can be considered to be the nucleophilic addition of water to the epoxide under acidic conditions. Reduction of an epoxide with lithium aluminium hydride and water generates an alcohol. This reduction process can be considered to be the nucleophilic addition of hydride (H-) to the epoxide under basic conditions. Reduction with tungsten hexachloride and n-butyllithium generates the alkene. This reaction in effect is a de-epoxidation. polyethers ] Johnson-Corey-Chaykovsky reaction In the Johnson-Corey-Chaykovsky reaction epoxides are generated from carbonyl groups and sulfonium ylides. Reactions Typical epoxide reactions are listed below. Nucleophilic addition to an epoxide can be base or acid catalyzed. 4 Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocation, i.e. the most substituted carbon (similar to a halonium ion). Under basic conditions, the nucleophile attacks the least substituted carbon, in accordance with standard SN2 nuclephilic addition reaction process. Hydrolysis of an epoxide in presence of an acid catalyst generates a glycol. The hydrolysis process of epoxides can be considered to be the nucleophilic addition of water to the epoxide under acidic conditions. Reduction of an epoxide with lithium aluminium hydride and water generates an alcohol. This reduction process can be considered to be the nucleophilic addition of hydride (H-) to the epoxide under basic conditions. Reduction with tungsten hexachloride and n-butyllithium generates the alkene. This reaction in effect is a de-epoxidation.