File
advertisement

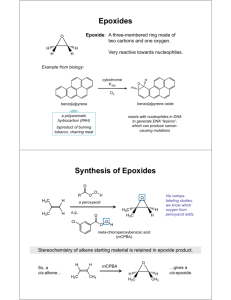
Sample Typed Report (for Diels Alder, E.A.S, Fischer and Aldol labs) The typed writeup represents a formal report to the scientific community on your experiment. In doing this, we are mimicking how research is reported in scientific journals. A brief introduction should be included, with structures drawn in Chemdraw. The experimental should be written in past-tense, third-person passive voice. All data relevant to the analysis of the product should be listed in a formal way, as shown below, followed by a brief conclusion. Synthesis of an Epoxide using mCPBA Mrs. Lisa Nichols, December 27, 2014 Introduction Alkene 1 was reacted with meta-chloroperoxybenzoic acid (mCPBA) to produce epoxide 2. O CN mCPBA O O CN racemic CH2Cl2 1 2 Experimental Alkene 1 (clear oil, 0.3170 g) was dissolved in dichloromethane (20 mL) in a round-bottomed flask equipped with a magnetic stir-bar. mCPBA (white solid, 1.39 g) was slowly added over a period of 15 minutes, and the reaction was stirred at room temperature for another hour. The reaction was quenched by the addition of aqueous sodium hydroxide (2%, 25 mL), and the layers were separated. The water layer was extracted with dichloromethane (3 x 10 mL). The combined organic layers were washed with saturated NaCl (20 mL) and dried over MgSO4. The drying agent was filtered, and the dichloromethane was removed on the rotary evaporator. The resulting solid was recrystallized from 8 mL of a 50% ether-50% hexane solution to give 0.2669 g of epoxide 2 (white solid). m.p. (Thiele tube): 65.2 – 68.0 ˚C. TLC (6:1 hexanes:ethylacetate): Rf = 0.19, purple with p-anisaldehyde stain (major spot); Rf = 0.29, UV active, brown with p-anisaldehyde stain (minor spot). GC (80C, ramp 5C/min to 120C): 1.23 min (6.5%, alkene 1), 5.45 min (93.5%, product 2). MS (70 eV): 27 (35), 41 (25), 57 (52), 73 (61), 86 (100), 113 (10) amu. IR (neat): = 3005 (s), 2952 (s), 2301 (w), 1254 (m), 1107 (s) cm-1. H NMR (300 MHz, CDCl3): = 4.34 (1H, d, J = 16.1 Hz), 4.33 (1H, d, J = 16.1 Hz), 3.97 (1H, dd, J = 11.5, 2.5 Hz), 3.48 (1H, dd, J = 11.5, 6.2 Hz), 3.19 (1H, ddt, J = 6.1, 4.2, 2.6 Hz), 2.84 (1H, dd, J = 4.8, 4.2 Hz), 2.65 (1H, dd, J = 4.8, 2.7 Hz) ppm 1 C NMR (75 MHz, CDCl3): = 116, 73, 55, 50, 44 ppm 13 Conclusion Epoxide 2 was synthesized from alkene 1 using mCPBA. 2 was 93.5% pure by GC and produced in a 67.6% yield. The structure of 2 was supported by melting point, TLC, IR, 1H NMR, and 13C NMR spectroscopy.