Supplemental Information
advertisement
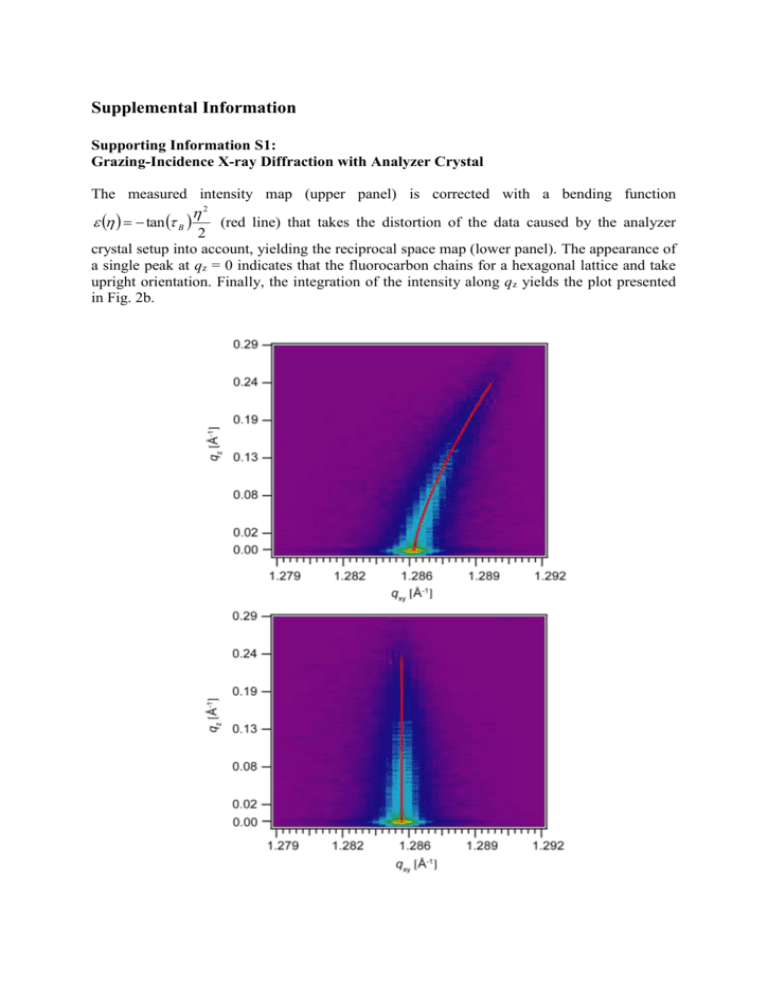
Supplemental Information Supporting Information S1: Grazing-Incidence X-ray Diffraction with Analyzer Crystal The measured intensity map (upper panel) is corrected with a bending function 2 tan B (red line) that takes the distortion of the data caused by the analyzer 2 crystal setup into account, yielding the reciprocal space map (lower panel). The appearance of a single peak at qz = 0 indicates that the fluorocarbon chains for a hexagonal lattice and take upright orientation. Finally, the integration of the intensity along qz yields the plot presented in Fig. 2b. Supplemental Information S2: Comparison to Yukawa Potential Effective interactions between nano-domains, compared to Yukawa potential (solid A line), w(r ) exp r . Note that the distance r is normalized by the domain size L. As r suggested by Fig. 4a, uncorrelated effective interactions that follow Yukawa potential can be observed only at [FL17] = 5 mol%. Supporting Information S3: Synthesis of Fluorinated Lipids The double chain fluorinated lipid, named FL17 was prepared by the synthetic sequence outlined below. In the first step, the formation of the fluorinated glycidol was performed in biphasic conditions (toluene-water) under phase-transfer catalysis. Consequently, the ring opening reaction took place in solid phase, under microwaves and afforded the corresponding bicatenar lipid as major product. 1-O-(1H,1H-perfluorooctadecyl) glycidol (F17): A mixture of 1H,1H-perfluoro-1octadecanol (3.7 mmol), 50 % aqueous NaOH (0.84 g, 20.9 mmol, 1.7 mL H2O), tetrabutylammonium bromide (0.079 g, 0.25 mmol), and benzene (5 mL) was stirred at room temperature for 15 min. Epichlorhydrin (2 mL, 26 mmol) was then added and the reaction mixture heated to 75 °C for 18 h. The reaction mixture was than cooled to 0°C, filtered and washed with water. The white solid was then dried over night. Yield 70%. 1H-NMR (CDCl3): 2.56 (dd, 1H, CH2 epoxide, J=4.6, 2.7 Hz); 2.75 (t, 1H, CH2 epoxide, J=4.6 Hz); 3.10 (m, 1H, CH epoxide); 3.46 (dd, 1H, CH2-O, J=11.8, 5.9 Hz); 3.90 (dd, 1H, CH2-O, J=11.8, 2.5 Hz); 3.99 (t, 2H, CH2-CF2, J=13.5); 13C-NMR (CDCl3): 44.04 (CH2 epoxide); 50.83 (CH epoxide); 68.52 (t, CH2-CF2, 2J(C,F)=26 Hz); 73.48 (CH2-O); 19F-NMR (CDCl3): -81.30 (t, 3 J(F,F)=13.05, CF3), -119.97 (CF2-CH2), -122.26, -123.21, -123.50 (all the others CF2), 126.64 (CF2-CF3). EI: m/z=956 [MH]+; m/z=913 (C17F35CH2OCH2+). Elemental analysis: C21H7F35O2, th. %C: 26.38, %H: 0.74, exp. %C: 25.18, %H: 0.98. 1,3-bis-O-(1H, 1H-perfluorooctadecyl) glycerol (FL17): A mixture of 1-O-(1H,1Hperfluorooctadecyl) glycidol (F17) (1g, 1.6 mmol), 1H,1H-perfluorooctadecanol (0.91 g, 3.5 mmol) and potassium methoxide (40 mg, 0.54 mmol) was heated to 100 °C under N2 and microwaves (P=300 W) for 2 h. The reaction mixture was than cooled to room temperature and added in 100 mL THF. The mixture was maintained under stirring for 2h, then the solution was filtered and the solvent evaporated. The product was purified by several recristalisations in CH2Cl2. Yield 36%. 1H-NMR (CDCl3/Freon F-113): 3.70-3.87 (d, 4H, CH2-CH-OH, J=4.9 Hz); 3.96-4.14 (2t, 4H, CH2-CF2, J=13.5 and 1H, CH-OH); 13C-NMR (CDCl3/Freon F-113): 67.95 (t, CH2-CF2, 2J(C,F)=26.5 Hz); 72.63 (CH-CH2-O); 76.62 (CHOH). Elemental analysis: C39H10F70O3, th. %C: 25.23, %H: 0.54, exp. %C: 23.48, %H: 1.17. [1] W. Huang, C. Jin, D. K. Derzon, T. A. Huber, J. A. Last, P. P. Provencio, A. S. Gopalan, M. Dugger, D. Y. Sasaki, J. Colloid Interf. Sci. 2004, 272, 457.