Topics for Chemistry 321 final
advertisement

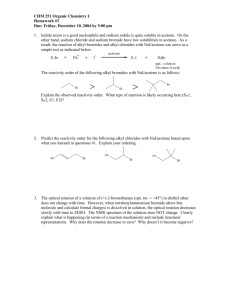
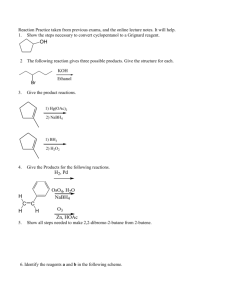
Topics for Chemistry 321 final. 1. We will cover material through Chapter 10, Section 10.7 (page 414). Topics you will need to know well are: 1. Bonding and Structure Lewis structures draw Lewis structures assign formal charges where appropriate resonance recognize major and minor contributors allyl systems delocalization of a lone pair from a heteroatom aromatic rings, etc. stabilization increases with number of equivalent contributing structures molecular orbitals , orbitals assign hybridization to atomic orbitals used to make bonds. molecular shape and polarity determine shape at any site in a molecule determine polarity of bonds in a molwecule determine molecular polarity reaction mechanisms be able to "push arrows" to indicate electron movement interconvert resonance contributors show movement of electrons during reaction 2. Acid/Base Chemistry Lowry-Brønsted acids/bases Ka, pKa order of acid strength strong/weak acids strong/weak bases conjugate acids/bases Lewis acids/bases electrophiles nucleophiles 3. Nomenclature functional groups know alkanes, alkenes, alkynes, dienes, alcohols, alkyl halides in depth. main chain vs substituents – endings!!! 4. Shapes of molecules – Stereochemistry conformations of ethane staggered, eclipsed conformations of butane anti, gauche, eclipsed conformations of cyclohexane chair, boat, skew axial, equatorial stereochemistry geometric isomers cis/trans, E/Z enantiomers R/S diastereoisomers recognize meso compounds stereochemistry of newly-introduced chiral centers when do you get diastereisomers, when do you get a racemate? 5. Alkene Chemistry reaction energy diagram reactants and products reactive intermediates activated complexes/transition states reaction coordinate rate determining steps electrophilic addition mechanism two steps carbocation intermediate potential for cation rearrangements stabilization of carbocations hyperconjugation conjugation with a bond (alkene, alkyne, aromatic ring) conjugation with a lone pair Markovnikov's Rule for addition HCl, HBr, HI, H2O/H+, etc. effects of lone pairs on electrophilic atom Markovnikov regiochemistry anti stereochemistry absence of free carbocation Br2/H2O; ICl; IN3; Cl2/H2O; Br2/CH3OH; etc. free radical addition of HBr – anti-Markovnikov addition. mechanism for this addition. hydration oxymercuration-demercuration stereospecific anti addition through mercurinium ion in step 1 loss of stereochemistry through free radical in step 2. Markovniokov regiochemistry no rearrangements hydroboration-oxidation stereospecific syn addition anti-Markovnikov regiochemistry catalytic hydrogenation syn addition of hydrogen 6. Alkyne Chemistry electrophilic addition acid-catalyzed hydration Hg2+ catalyst required Markovnikov regiochemistry enol intermediate keto-enol tautomerization addition of 1 mole of halogens anti stereochemistry addition of 2 moles of electrophile – Markovnikov addition catalytic hydrogenation H2/Pt gives alkane H2/Pd-BaSO4/PbSO4/quinoline ("Lindlar palladium") gives Z alkene Birch reduction Na/NH3 or Li/NH3 gives E alkene acidity of terminal alkynes RCC—H has pKa ≈ 26 alkynide (acetylide) ions are strong nucleophiles use as nucleophiles in SN2 reactions. use of alkynes in synthesis. 7. Diene Chemistry electrophilic addition 1,2- vs. 1,4-addition kinetic vs thermodynamic control Diels-Alder reaction [4+2] cycloaddition product is a cyclohexene diene reacts with dienophile (alkene) electron-withdrawing groups on alkene accelerate reaction conjugating groups on alkene accelerate reaction stereospecific E alkene gives E cyclohexene product Z alkene gives Z cyclohexene product E,E-diene and Z,Z-diene give Z cyclohexene product E,Z-diene and Z,E-diene give E cyclohexene product alkynes may be sued as dienophiles in Diels-Alder reactions. 8. Free Radical Chemistry definition of a radical species with at least one unpaired electron stability of radicals 3° > 2° > 1° > Me allyl, benzyl > 3° (usually) radical chain reactions all product is formed in propagation steps only one product is formed in each propagation step chain carriers formed in initiation step must be present for reaction to continue are free radicals themselves "reaction cycle" diagram showing chain carriers allylic halogenation N-bromosuccinimide replacement of allylic hydrogen by halogen allyl radical intermediate resonance contributing forms show where reaction will occur 9. Nucleophilic Substitution in Alkyl Halides mechanism 1-step: SN2 2-step: SN1 carbocation intermediate rate determining step stereochemistry: SN2: inversion SN1: partial or total racemization effects of nucleophile strength strong nucleophiles: RS—, RO—, I—, CN—, N3—; etc. modest nucleophiles: R3N, Br—; etc. weak nucleophiles: H2O, ROH, etc. strong nucleophiles favor SN2, weak nucleophiles favor SN1 effects of solvent protic solvents favor reactions that go through more ionic states (SN1) aprotic dipolar solvents favor reactions that go through charge-dispersed transition states DMF, DMSO, HMPA aprotic dipolar solvents accelerate SN2 reactions dramatically. effects of alkyl group structure SN2: allyl, benzyl > 1° > 2° >> 3° SN1: allyl, benzyl > 3° > 2° >> 1° effects of leaving group identity I > Br > Cl >> F 10. Elimination Reactions of Alkyl Halides mechanism 1-step: E2 2-step: E1 carbocation intermediate rate determining step stereochemistry: E2: proceeds anti E1: most stable stereoisomer of alkene usually predominates regiochemistry E1: Zaitsev's Rule predicts major product (most substituted alkene predominates) E2: Zaitsev's Rule predicts stereochemistry if more than one anti path allowed. effects of alkyl group structure E1 and E2: 3° > 2° >> 1° effects of leaving group identity I > Br > Cl >> F