Microsoft Word - Final Exam Study Guide
advertisement

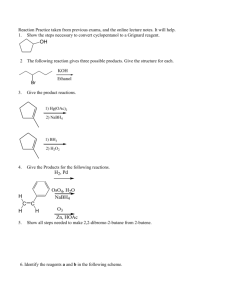
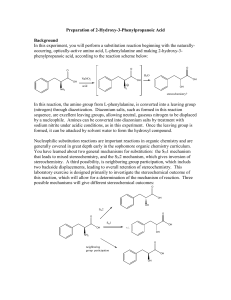
C341 Final Exam Study Guide Summer 2015 General information: 8:00 AM- 11:00AM on Friday, july 31 in BH013 Format: 200 points, cumulative, similar format to other exams Approximate point distributions: ~1/3 mechanism Draw the arrow mechanism (substitution, elimination, addition, rearrangements, redox) Answer questions based on mechanisms ~1/3 reagents, reactions Predict the major product with stereochemistry and regiochemistry Provide the correct reagents Multistep synthesis ~1/3 miscellaneous concepts from list below Tips for studying: Work from the end of the course to the beginning (study backwards). Emphasize chapters 7-10 and 12-14. Chapters 1-6 were the foundation for these chapters. Print blank discussion problem sets and write full answer before checking key Retake old exams and quizzes, then check your answers Do practice chapter problems not listed under Daily Homework A list of major topics (not all inclusive) Formal charge, resonance structures, hybridization, bond-line structures, acid/base equilibria, pKa’s, trends in acidity/basicity, functional groups, alkane nomenclature, conformational analysis, Newman projections, causes of strain, cyclohexane ring structures, chiral, achiral, R/S nomenclature, enantiomers, diastereomers, optically active, meso compounds, arrow mechanisms, transition states, rate limiting steps, activation barrier, exothermic/endothermic reactions, E/Z nomenclature, addition reactions, Markovnicov’s rule, anti-marcovnicov product, regiochemistry of additions, stereochemistry of additions, reactions of alkynes, acidity of terminal alkynes, calculating change in energy for reaction, Hammond’s postulate, nucleophile, leaving group, substitution reactions, SN1 mechanism, SN2 mechanism, alkene classifications, alkene stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesis, protecting groups, redox reactions, reagents for redox reactions, Grignard reaction \ 1. Mechanisms. These are the very basic types of mechanisms. You should also be able to explain regiochemistry and stereochemistry outcomes, as well as rearrangements, etc. You should be able to apply these basic mechanisms to more challenging molecules, or to explain why an expected result does not happen. 2. Structure (formal charge, hybridization, aromatic, resonance, stereochemistry, conformational analysis) A. Answer the following questions pertaining to this structure. O H N A. How many hydrogens? How many lone pairs? B. Label the structure with any missing formal charges. C. Draw at least two more resonance structures and rank them from most major contributor to most minor contributor. D. Mark all the chiral centers and label any designated chiral centers R or S. B. Would you expect the elimination of cis-1-t-butyl-4-chlorocyclohexane using a strong base to be faster or slower than the elimination of chlorocyclohexane with a strong base? 3. Acid/base reactions For each of the following compounds, circle the most acidic hydrogen. Give an approximate pKa value. Would this compound be deprotonated by HO-? Would this compound be deprotonated by H2N-? Draw the conjugate base of each acid. A. B. O C. O OH O 4. Predict the MAJOR products. Include stereochemistry where needed.