CHM 251 Organic Chemistry I
advertisement
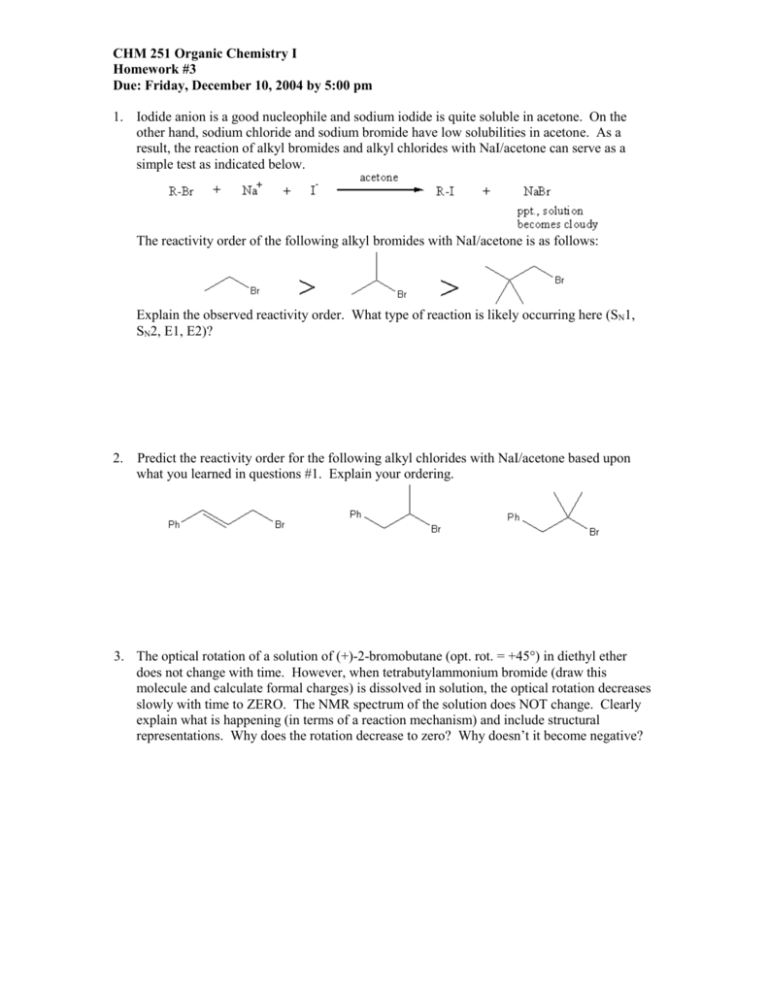
CHM 251 Organic Chemistry I Homework #3 Due: Friday, December 10, 2004 by 5:00 pm 1. Iodide anion is a good nucleophile and sodium iodide is quite soluble in acetone. On the other hand, sodium chloride and sodium bromide have low solubilities in acetone. As a result, the reaction of alkyl bromides and alkyl chlorides with NaI/acetone can serve as a simple test as indicated below. The reactivity order of the following alkyl bromides with NaI/acetone is as follows: Explain the observed reactivity order. What type of reaction is likely occurring here (SN1, SN2, E1, E2)? 2. Predict the reactivity order for the following alkyl chlorides with NaI/acetone based upon what you learned in questions #1. Explain your ordering. 3. The optical rotation of a solution of (+)-2-bromobutane (opt. rot. = +45°) in diethyl ether does not change with time. However, when tetrabutylammonium bromide (draw this molecule and calculate formal charges) is dissolved in solution, the optical rotation decreases slowly with time to ZERO. The NMR spectrum of the solution does NOT change. Clearly explain what is happening (in terms of a reaction mechanism) and include structural representations. Why does the rotation decrease to zero? Why doesn’t it become negative? 4. Explain the following observation: 5. When 4-chloro-2-methyl-2-pentene reacts with acetic acid (solvent), two substitution products are formed with one product predominating versus the other. When small amounts of the acetate anion are added to the reaction mixture, no change in rate is observed. (a) The minor isomeric product can actually exist in two different stereochemical forms as a result of the course of this reaction. What are the structures corresponding to each of these products and what is the stereochemical relationship between the two? Are they likely to be formed in equal quantities? relationship stereoisomer #1 stereoisomer #2 (b) Provide a complete reaction mechanism that explains how the two different stereoisomers of the minor product are formed. (c) In part (b), you should have generated a key intermediate structure. This is not the most stable form of this intermediate. SHOW (using the curved arrow formalism) how this intermediate alters itself to achieve a more stable configuration of groups. If resonance is involved, be sure to draw all of the possible resonance contributors. How does this affect the percentage of regioisomers formed (major vs. minor)? starting intermediate ending intermediate (d) Overall, what is the reaction that is occurring here (SN1, SN2, E1, E2) and why does the addition of small amounts of the acetate not affect the rate of reaction? 6. Complete the table below in which you will rank the carbocations shown in DECREASING order of stability. Be sure to indicate the type(s) of special effects that are contributing to the relative stability of each ion. For those ions that exhibit a resonance stabilization effect --show ALL possible resonance structures. Order of stability (most stable) = (least stable) = Stabilizing Effects (Reason for ranking) 7. Given the following incomplete reaction: O (2S,4S)-2-iodo-4-methylhexane H3C O Na acetone (a) Draw a skeletal structure for the reactant in the reaction showing the correct stereochemistry. (b) (c) (d) (e) Draw a CIRCLE around the nucleophile in the reaction. Draw a SQUARE around the best leaving group in the reaction. Place a CHECK MARK next to the any electrophilic carbons. Is the leaving group attached to a primary, secondary, or tertiary carbon? (f) Do you have a good leaving group or poor leaving group? (g) Is your nucleophile a strong base or weak base? (h) Do you have a good nucleophile or a poor nucleophile? (i) What type of solvent is used in this reaction – PAD or PPD (See SAM pg. 10)? Why do we want to use this type of solvent in this case? (j) Given your answers to parts (e-i), do you think this reaction is more likely SN1 or SN2? (k) Provide a mechanism showing all relevant transition states/intermediates for the reaction chosen. (l) Provide skeletal structure(s) of the expected major product(s). (m) Draw a fully labeled reaction energy diagram that is consistent with your chosen type of reaction Be sure to answer the following questions AFTER drawing your energy diagram. (a) Which species does the first transition state resemble more closely? (b) Which species does the second transition state resemble more closely? (c) Which step describes the rate-determining step? (d) Use the bond dissociation energies listed pages 36 and 355 of your book to estimate the H for the reaction. Use this value to calculate the Keq for this reaction. Is the reaction exothermic or endothermic as written? Explain. 8. The azide ion (N3-) is known to react by an SN2 mechanism thousands of times more rapidly with 2-bromopentane than with its constitutional isomer neopentyl bromide (1-bromo-2,2dimethylpropane), despite the fact that the leaving group is at a secondary carbon in the first compound and at a primary carbon in the second. Explain these facts by drawing/interpreting their structures. 2-bromopentane neopentyl bromide 9. To reach the conclusion that the reaction of the azide ion with 2-bromopentane in the previous problem (#8) did indeed occur as an SN2 reaction, the chemists studying the reaction did several additional experiments. Predict what the scientists would have observed in each experiment if the reaction really took place via an SN2 pathway. (a) they used optically active (R)-2-bromopentane (b) they doubled the concentration of the alkyl bromide (c) they doubled the concentration of the azide ion Even though the reaction shown below can potentially give two stereoisomeric elimination products, only one is actually formed. OTs H Ph O-K+ Ph H CH3 (CH3)3COH heat (a) Assign the stereochemistry (R/S) at each stereogenic atom in the starting material. (b) Provide the structure for the single alkene product in the box provided (HINT: you’ll need to use your model kit). (c) Two possible diastereomers of the tosylate shown above also react with potassium tbutoxide to give an alkene. Provide the structure of one of the two diastereomers in the box provided with assigned (R/S) stereochemistry at each stereogenic atom. O-K+ (CH3)3COH heat diastereomeric tosylate diastereomeric alkene (d) Provide the structure of this (new) alkene product in the box provided that is diastereomeric to the alkene product formed above (HINT: again use your model kit). (e) Clearly account for the stereochemical course of these two reactions. Why is there no cross-over? Explain using a structural model.