The Synthesis and Characterization Of Ferrocene
advertisement

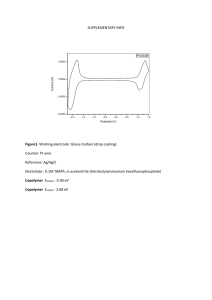
The Synthesis and Characterization of Ferrocene Courtney Arnott October 1, 2001 Background Ferrocene is an extremely stable organometallic compound with the formula Fe(C5H6)2. Pure ferrocene has the form of orange-yellow, flaky crystals with a melting point of 172-174°C, and a molecular weight of 186g. It is soluble in organic solvents and insoluble in water.1 Ferrocene was first synthesized in 1951, and IR and NMR data predicted its unusual structure – two 5-carbon rings with a central iron atom attached to the pi bonds of the rings. The structure was confirmed in 1954 through the use of x-ray crystallography.2 The commercial applications of ferrocene include a fuel additive, a catalyst for rocket propellant, a curing agent for silicon resins and rubber, and a raw material for antibiotics or blood products.3 The reaction of cyclopentadiene to ferrocene is shown in Equation 1 below.4 (1) Acetylferrocene (ferrocenyl methyl ketone, m.w. 228g) is a ferrocene molecule with an acetyl group off of one carbon ring. It is a highly toxic, orange crystalline compound with the structure C5H5FeC5H4COCH3. Acetylferrocene has a melting point of 85-86°C.5 Experimental Reagents Synthesis of ferrocene: 15g KOH (Allied Chemical), 30mL 1,2-Dimethoxyethane (Sigma), 2.75mL cracked cyclopentadiene (Aldrich), 3.25g FeCl2 4H2O (Fisher Scientific), 12.5mL DMSO (Sigma), 45mL 6M HCl (VWR Scientific Products), 100mL ether (VWR Scientific Products) Synthesis of acetylferrocene: 1g ferrocene (see supplemental materials for calculations of amount corrections), 3.33mL acetic anhydride (Fisher Scientific), 0.66mL 85% H3PO4 (Fisher Scientific), 10g CaCl2 (EM Science) Purification of acetylferrocene: 20mL petroleum ether (VWR Scientific Products), 20mL toluene (Mallinckrodt), 20mL ethyl ether (Aldrich), 20mL ethyl acetate (Mallinckrodt), 2mL ethyl acetate + 18mL petroleum ether*, 10mL ethyl acetate + 90mL petroleum ether* * see supplemental materials for amount calculations Workup and Analysis All ferrocene synthesis reactions were carried out under a N2 atmosphere using standard Schlink techniques. The ferrocene product was dried in a Buchi Rotavapor R114 rotary evaporator and sublimated on a vacuum cold finger. Melting points were taken with a Fisher-Johns melting point apparatus. Cyclic voltammetry data was obtained with a EG&G Princeton Applied Research Potentiostat/Galvanostat Model 263A, using a silver chloride wire as the reference electrode, a platinum wire as the working electrode, and a tungsten wire as the counter electrode. IR spectra were taken with a Mattson Instruments 4020 Galaxy Series FT-IR as KBr pellets. Purification of the acetylferrocene sample was done by silica gel flash chromatography using a solution of 90% petroleum ether and 10% ethyl acetate. Synthesis Synthesis of ferrocene and acetylferrocene followed the procedure of Tanner et al6. An alternative collection method, described below, was used to obtain the synthesized ferrocene product. Ether was added to extract the organic material from the water, and the bottom (water) layer was filtered off with a sep funnel. We then tried (unsuccessfully) to oxidize the product in the water layer with zinc powder. The ether layer was filtered through a frit packed with celite to remove the debris, and then filtered through a frit packed with MgSo4 to remove excess water. The product, suspended in ether, was dried in a rotary vacuum and collected from the inside of the flask. The product was sublimated to collect the pure ferrocene crystals. The next step in the procedure was to synthesize acetylferrocene, also according to Tanner et al6. Thin layer chromatography (TLC) was used to determine the best solvent for purifying the acetylferrocene in a silica flash column. A 10%-90% mixture of ethyl acetate and petroleum ether showed the best separation. Half of our acetylferrocene (dissolved in the solvent) was run through the silica column and the first band of pure acetylferrocene was collected; the other half was run though a fritted funnel with silica gel to save time. Both solutions of pure acetylferrocene were poured into watchplates to evaporate and crystallize. Results/Conclusions 0.2453g of pure ferrocene was obtained after sublimation; 0.9406g was obtained after collection according to the methods of Tanner et al6 (1.186g total). The total yield of acetylferrocene was 0.1285g. We obtained a 38.32% yield of ferrocene and a 10.44% yield of acetylferrocene (see supplemental materials for % yield calculations). Characterization of both products was conducted by taking the melting points and IR spectra of both ferrocene and acetylferrocene, and obtaining the cyclic voltammetry data on ferrocene. The FTIR data of acetylferrocene showed the same peaks as the ferrocene spectra. We did not see the sharp C-O stretch (singlet) at 1670-1710. This data showed that the acetylation failed. In our cyclic voltammetry data, our peak-to-peak separations for ferrocene were 106.5 for our first run and 763.7 for our second run. On the second run, a tungsten wire was used as the reference and counter. This run was smoother, but it showed a trace of impurity with an extra bump in the graph. The top peak for the first run was 0.324V, the bottom peak was 0.431V, and the E0 obtained experimentally was 0.387V. This is comparable to the published literature value of 0.27V. The melting point of ferrocene was 155-160°C. The published melting point was 172-174°C. This difference could be due to some impurities in the ferrocene. We were unable to obtain the melting point of acetylferrocene – some small amount of orange crystal melted at 140°C (much higher than the melting point of 85-86°C) but most of our product was a light brown powder that did not melt. This could possibly be due to impurities in the sample, which was actually ferrocene (the orange crystals) since the IR data showed we were unsuccessful in synthesizing acetylferrocene. References 1. Harrison, Karl. “Ferrocene - Synthesis.” http://www.ncl.ox.ac.uk/mom/ferrocene/ synthesis.html. University of Oxford, 1996. 2. Harrison, Karl. “Ferrocene – Molecule of the Month, June 1996.” http://www.ncl. ox.ac.uk/mom/ferrocene/ferrocene.html. University of Oxford, 1996. 3. “Ferrocene.” http://www.americanlb.com/ferrocene.html. 4. Harrison, Karl. “Ferrocene - Synthesis.” 5. Chempros.com. “Chemical Dictionary – A.” http://www.chempros.com/ knowledgebase/abate.htm. 2000. 6. Tanner, Pamela S. et al. “The Synthesis and Characterization of Ferrocene.” State University of New York, Binghamton. http://imr.chem.binghamton.edu/labs/ ferrocene/ferrocene.html. New York, 1995. Supplemental Materials Theoretical/Percent Yields 2.75mL cyclopentadiene x .8g/mL = 2.2g x 1 mol/66g = .033 mol .033 mol x 1 mol ferrocene = .0167 mol (theoretical yield) 2 mol cyclopentadiene 1.186g ferrocene x 1 mol/186g = .0064 mol (actual yield) .0064 mol x 100 = 38.32% .0167 mol 1g ferrocene x 1 mol/186g = .0054 mol .0054 mol x 1 mol ferrocene = .0054 mol (theoretical yield) 1 mol acetylferrocene .1285g acetylferrocene x 1 mol/228 g = 5.64x10-4 mol (actual yield) 5.64x10-4 mol x 100 = 10.44% .0054 mol Correcting reactant amounts in synthesis of acetylferrocene 1 g ferrocene obtained = .66 1.5 g ferrocene needed (multiply all amounts by .66) 5 mL acetic anhydride * .66 = 3.33 mL acetic anhydride 1 mL H3PO4 * .66 = .66 mL H3PO4 Making solvent solution for TLC/silica column 20 mL total * 10% ethyl acetate = 2 mL ethyl acetate 20 mL total * 90% petroleum ether = 18 mL petroleum ether (We also used a solution of 10 mL ethyl acetate and 90 mL petroleum ether for the solution in our silica gel column)