Thin Layer and Column Chromatography
advertisement

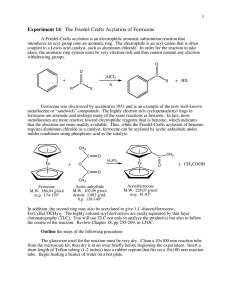
Thin Layer and Column Chromatography Experimental Procedure for Part 3: Purification of Ferrocene by Sublimation 1. Evaporate the solvent from the yellow ferrocene solution under vacuum. Use a steam bath if heat is required. 2. Determine the weight of the ferrocene residue. 3. To sublime the ferrocene residue, see Figure 2 in “Figures for Column Chromatography and Sublimation” under Resources. Push a centrifuge tube through a black neoprene adapter. Place it and the centrifuge tube into the mouth of the flask with contains the ferrocene. 4. Place the apparatus in a thermowell and clamp it so that the bottom of the flask is touching the sides of the thermowell. Attach the side-arm of the flask to the vacuum with black rubber tubing. 5. Turn on the vacuum. Next, fill the centrifuge tube with pieces of ice, so that the centrifuge tube becomes a “cold finger.” Finally, turn on the thermowell to a high setting. 6. Watch as the ferrocene sublimes onto the surface of the cold finger and the upper sides of the flask. When the bottom of the side-arm flask is empty of solid material, wrap the sides in aluminum foil so that all the ferrocene collects on the cold finger. 7. Turn off the thermowell, then the vacuum. Very slowly and carefully disconnect the rubber hose from the side-arm (a blast of air can knock the sublimate off the cold finger!) 8. Carefully, remove the centrifuge tube from the flask. Scrape the ferrocene off the tube and onto tared weighing paper. Record the weight. Take a melting point of the purified compound. 9. Evaporate the solvent from the acetylferrocene orange solution by heating the flask on a steam bath. Obtain the weight and melting point of the acetylferrocene.