Ferrocene Synthesis
advertisement

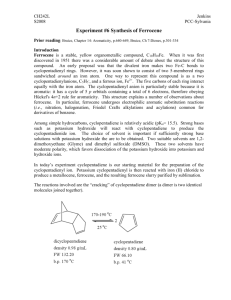
Experiment 1 Synthesis, Purification, and Characterization of Ferrocene Part A: Part B: Part C: Synthesis of Ferrocene Purification of Ferrocene Characterization of Ferrocene Introduction The compound ferrocene, or also known as [Bis(cyclopentadienyl)iron(II)], is prepared via the direct reaction of potassium hydroxide, cyclopentadiene, and iron (II) chloride tetrahydrate. 8 KOH + 2 C5H6 + FeCl2 * 4 H2O Fe(C5H5)2 + 2KCl + 6 KOH*H2O Total time required: 1 day (or 5 hours if product dried in vacuo) Actual working time: 4 hours Preliminary reading and techniques assignment: J. Birmingham, “Synthesis of Cyclopentadiene Metal Compounds,” Adv. Organometallic Chem., 2, 365 (1965); G. E. Coates, M. L. H. Green, and K. Wade, Organometallic Compounds, vol. 2, Methuen & Co. Ltd., London, 1968, pp. 90115; W. L. Jolly, Inorg. Chem., 6, 1435 (1967); and, W. L. Jolly, “The Synthesis and Characterization of Inorganic Compounds,” Chapter 5, Solvents, and Appendix 4, Compressed Gas Cylinders. Reagents Required: 11 ml of 1,2-dimethoxyethane About 5.2 g of KOH pellets (protected from moisture) 1 ml of cyclopentadiene – obtained from the thermal cracking of 2 ml dicylopentadiene (3a,4,7,7a-tetrahydro-4,7-methanoindene) 1.1 g of FeCl2 * 4 H2O 5 ml of dimethyl sulfoxide (DMSO) 5 ml of aqueous HCl About 20 g of ice Cylinder of nitrogen or argon Experimental Section Part A: Synthesis of Ferrocene Safety Recommendations (1) Potassium Hydroxide (CAS No. 1310-58-3): Poison! Danger! Corrosive. Causes severe burns to skin, Eyes, respiratory tract, and gastrointestinal tract. Material is extremely destructive to all body tissues. May be fatal if swallowed. Harmful is inhaled. (Refer to attached MSDS forms for further info) (2) Hydrochloric Acid (CAS No. 7647-01-0): Poison! Danger! Corrosive. Liquid and mist cause severe burns to all body tissue. May be fatal is swallowed or inhaled. Inhalation may cause lung damage. (Refer to attached MSDS forms for further info) (3) Dimethyl Sulfoxide (CAS No. 67-68-5): Warning! Harmful if swallowed, inhaled or absorbed through the skin. Causes irritation to skin, eyes, and respiratory tract. Combustible liquid and vapor. (Refer to attached MSDS forms for further info) (4) Dicyclopentadiene (CAS No. 77-73-6): May cause mild eye irritation, severe skin irritation, and is toxic if inhaled or ingested. Keep away from heat, sparks, and flames. (Refer to attached MSDS forms for further info) (5) Dimethoxyethane (CAS No. 110-71-4): Warning! Flammable liquid and vapor. Affects central nervous system. May affect blood and blood forming organs, reproductive system, liver and kidneys. May form explosive peroxides in air. Possible birth defect hazard. May cause birth defects based on animal data. May be harmful if absorbed through skin. May cause to eyes and respiratory tract. (Refer to attached MSDS forms for further info) (6) Iron (II) chloride tetrahydrate (CAS No. 13478-10-9): Danger! Corrosive. Causes severe irritation or burns to every area of contact. Harmful if swallowed or inhaled. Affects the liver. (Refer to attached MSDS forms for further info) (7) Cyclopentadiene (CAS No. 542-92-7): May cause eye irritation. May cause coughing and sore throat if inhaled. Flammable, keep away from heat, sparks, and flames. Above 25 explosive vapor/air mixtures may be formed. (Refer to attached MSDS forms for further info) Chemical Data Compound 1 2 3 4 5 6 7 FW 56.11 36.46 78.13 132.20 90.12 198.812 66.0 Amount 5.2 g 7.8 g 4.35 ml 3 ml 10.4 ml 1.1 g 1 ml mol 0.09 0.21 0.06 0.02 0.12 0.01 0.02 mp (C) 406 -114.18 19 -58 105 -85 bp (C) 1327 -85 189 172 82-83 1023 41.5-42 Density 2.044 1.600 1.1014 0.96 0.86 1.93 0.8 Required Equipment Fractional distillation apparatus 30 ml three-necked (standard taper joints) round bottomed flask Magnetic stirrer and large stirring bar T-tube mercury bubbler with standard-taper connection to flask Pyrex Petri dish with cover, 150 x 20 mm Experimental Procedure The potassium hydroxide pellets are ground with a mortar and pestle until the particles are less than 0.5 mm in diameter. Make sure that the potassium hydroxide has minimal exposure to the atmosphere, and stored in a tightly capped bottle. The cyclopentadiene is made from the thermal cracking of dicyclopentadiene. The dicyclopentadiene is slowly distilled in the hood through a fractionating column, collecting only the material that refluxes below 44. (See Figure 4.2) After putting on some gloves, the magnetic stir bar, 11 ml of 1,2dimethoxyethane, and 5.2 g of the powdered potassium hydroxide are placed in the three-necked flask. (See Figure 32.12) One side neck is stoppered and the other is connected to the t-tube mercury bubbler and the nitrogen cylinder. While this mixture is slowly stirring, and being flushed by the stream of nitrogen, 1.0 ml of cyclopentadiene is added. The center neck is then fitted with the dropping funnel with its stopcock open. After about 99% of the air has been flushed from the flask, the stopcock is closed, and the solution of 1.1 g of FeCl2 * 4 H2O in 5 ml of DMSO is placed in the drop funnel. The mixture is stirred vigorously. After 10 minutes has passed, the t-tube is lifted above the level of mercury (to reduce pressure to atmospheric), and drop-by-drop addition of the iron(II) chloride solution is begun. The rate should be adjusted so that the entire amount is added in 45 min. After the complete addition, the stopcock is closed and vigorous stirring is continued for 30 min. Finally the nitrogen flow is stopped, and the mixture is added to 5 ml of 6 M HCl and about 20 g of crushed ice. The resulting slurry is stirred for about 15 min, and the precipitate is collected on a sintered-glass funnel and washed with four 3.0 ml portions of water. The moist solid is spread out on a large watch glass and dried in the air overnight. The yield is approximately 1 g of ferrocene. Chemical Data Compound Ferrocene FW Amount 186.04 1 g Part B: mol 0.005 mp(C) 173-174 bp(C) 249 Density - Purification of Ferrocene An extremely pure product can be obtained by sublimation. The material to be sublimed is placed in an inverted cover of a Petri dish, making sure that none of the material is within 2 mm of the sidewall of the cover. The Petri dish itself (the smaller of the two pieces) is inverted and placed in the cover, and then the entire unit is placed on a hot plate. The hot plate is slowly warmed up until the surface of the unit is almost too hot to touch. After 4 to 10 hours, the ferrocene should be completely sublimed onto the upper glass surface and should be completely separated from the bottom residue layer. The yield is now approximately 0.9 g. (see Figure 32.13) Part C: Characterization of Ferrocene The infrared spectrum of ferrocene may be determined using a KBr pellet. Absorption bands are observed at the following frequencies (in cm-1): 170(m), 478(s), 492(s), 782(w), 811(s), 834(w), 1002(s), 1051(w), 1108(s), 1188(w), 1411(s), 1620(m), 1650(m), 1684(m), 1720(m), 1758(m), 3085(s) The ultra violet spectrum in ethanol show a maxima at 325 m (=50) and 440 m (=87) and rising short wavelength absorption (=5250 at 225 m) Another determination can be acquired through its melting point determination, melting at 173-174. References 1. 2. 3. J. Birmingham, “Synthesis of Cyclopentadiene Metal Compounds,” Adv. Organometallic Chem., 2, 365 (1965) G. E. Coates, M. L. H. Green, and K. Wade, Organometallic Compounds, vol. 2, Methuen & Co. Ltd., London, 1968, pp. 90115 W. L. Jolly, Inorg. Chem., 6, 1435 (1967) General References 1. W. L. Jolly, “The Synthesis and Characterization of Inorganic Compounds,” Chapter 5, Solvents, and Appendix 4, Compressed Gas Cylinders.