FNF II Lesson 28 Lab
advertisement

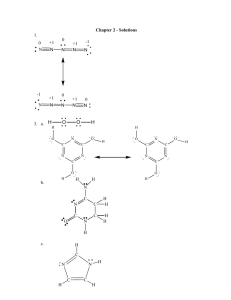
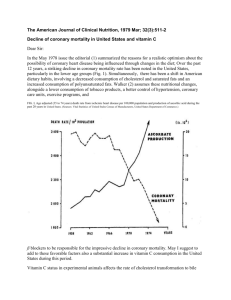
Investigations in Biology and Chemistry II – Lab Work Name: _____________________________________ Point Value: 145 Date Due: ________ Points for Completion: Name, Title, Date, Hypothesis, Data/Results (6), Significant Figures(3), Neatness, Safety Precautions (total possible pts for Completion: 21) Writing Rubric: Assessed and graded at “Exceeds Standard” point value of 24 Lab Rubric Item: Understanding the Purpose of the Experiment #1A or 1B— Assessed and Graded at “Exceeds Standard” point value of 20 Lab Rubric Item: Understanding the Purpose of the Experiment #2—Assessed and Graded at “Exceed Standard” point value of 20 Lab Rubric Item: Understanding the Purpose of the Experiment #3— Assessed and Graded at “Exceeds Standard” point value of 20 Lab Rubric Item: Organizing and Presenting Data #1-4—Assessed and Graded at “Exceeds Standards” point value of 40 (total possible point for Rubric Items: 100) Lab #34: Investigating the Effects of Heat on Ascorbic Acid Pre-Lab Activities 1. Read the Introduction up to, but not including, the subsection The Behavior of Vitamin C, completing the tasks as you go. Now go back and re-read those same pages, this time highlighting main ideas. 2. Set-up your lab notebook for Lab #34. 3. Interactive Lecture: What is oxidation? 4. Finish reading the Introduction. Remember to complete the tasks and highlight the main ideas. 5. Read the Materials and Methods section up to, but not including, the subsection The Reduction of Ascorbic Acid by DPIP. Remember to complete the tasks and highlight the main ideas. 6. Interactive Lecture: What is Vitamin C’s role in the human body? 7. Finish reading the Materials and Methods section. Remember to complete the tasks and highlight the main ideas. 8. Read through the Procedure and circle any new or unfamiliar materials. Access the MSDS for appropriate circled materials. Flinn Scientific Homepage: http://www.flinnsci.com: a) Click on Safety on the menu to the left; b) select MSDS from the menu on the next page; c) click on the letter of the material you are researching from the alphabet provided. Record any important information, such as health hazards or emergency procedures, in the space below: 9. Interactive Lecture: What is an oxidation-reduction, or redox, reaction? Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 1 of 18 10. Transfer any Safety Precautions to your lab notebook. 11. Based on information in the Materials and Methods and Procedure sections, calculate the molarity of the stock solution of ascorbic acid. Use the factor-label method. You can do this in your lab notebook. 12. Based on the information in the Materials and Methods and Procedure sections, calculate the molarity of each of the dilutions made from the stock solution. You can do this in your lab notebook. Make a notation next to the calculation for the dilution that your lab team will use in the experiment. 13. Based on your responses to #10, determine the grams of ascorbic acid in each of the dilutions. You can do your calculations in your lab notebook. 14. In your lab group, create a hypothesis that addresses the effect of heat on levels of ascorbic acid in juice samples. Record your hypothesis in your lab notebook. 15. Interactive Lecture: What is the purpose of the Introduction section of a scientific paper? 16. Reread the entire Introduction and Materials and Methods sections, revisiting any tasks you have not completed. In addition, review the Procedure. 17. Interactive Lecture: What is the purpose of the Results section of a scientific paper? 18. Review the Procedure and construct the necessary data tables in your lab notebooks. 19. Familiarize yourself with the new lab techniques during your Lab Technique Discussion. Record any notes next to the appropriate Procedure steps. 20. In this lab, you will be writing up consecutive Rubric Items from the Understanding the Purpose of the Experiment Items. These items will flow together in a well-written series of paragraphs. Refer to The Science Writing Rubric. These writing conventions will now be graded at an Exceeds Standards point value of 24. Refer to your Lab #34 Peer Review Sheet and write your Performance Improvement Plan (PIP) under Writer’s Directions. In addition, you also will apply your skills for Organizing and Presenting Data # 1-4 by constructing a Results section that will be assessed at an “Exceeds Standard” point value of 40. Using your updated Lab Rubric Summary Sheet, find the graded labs in your notebook where you previously addressed these Rubric Items to see how well you performed on each one—what can you do to better apply these skills? Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 2 of 18 21. Examine the Pre- & Post-Lab Activities, as well as tasks embedded in the Introduction and Materials and Methods, indicating which activities connect to the Lab Rubric Items addressed in this lab. This will help you “Achieve” or “Exceed Standard.” Introduction Sources of Vitamin C Vitamin C is a water-soluble vitamin. It is also known as ascorbic acid, an organic acid with the formula C6H8O6 (Figure 1). Vitamin C is essential for normal body function, though humans do not have the ability to synthesize it internally since we do not possess a critical enzyme that helps catalyze the corresponding synthetic reactions. Interestingly, our ancestors had the gene that coded for this enzyme, but mutagens caused changes in the sequence that rendered the gene ineffective in producing this enzymatic protein after that time. Fortunately, the environment of many of our human ancestors was our collective friend. The habitats of these primates provided many ingestible sources of vitamin C. What might have happened if food sources containing vitamin C were unavailable during this time in Earth’s history? Record your thoughts in the space provided. As descendants of these primates, human beings must obtain vitamin C through their diet. The recommended daily allowance (U.S. RDA) is 65 mg of vitamin C for adolescent females ages 15-18 and 75 mg for adolescent males ages 15-18. Note that after age 18, adult females should increase their daily intake to 75mg and adult males to 90 mg. Download the reference table from http://www.iom.edu/Object.File/Master/21/372/0.pdf for more information about RDA. Great dietary sources of vitamin C include red peppers and kiwis; additional sources may be found in Table 1. Reflect on your own diet. In the space provided, write down your three primary vitamin C-containing food sources. They could be foods from the Table 1 or from items that you know you consume on a daily basis, such as vitamin C-fortified apple juice. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 3 of 18 Primary Sources of Vitamin C in My Diet: Functions of Vitamin C in the Human Body You may know that vitamin C helps the immune system fend off the common cold and sore throat. Vitamin C is a key player in immune system function because it enhances the body’s ability to fight infections and decreases cancerous tumor formation. Specifically, vitamin C helps stimulate antibody production as well as the production of certain white blood cells. If ingested alongside a food containing iron, vitamin C also helps the body absorb iron, which may help prevent anemia. For people with allergies, ensuring the RDA for vitamin C may help ease reactions, since moderately high levels of vitamin C can block the release of, and break down, histamines. In certain kinds of allergic reactions, histamines flood the system in a “false immune response.” Some scientists believe that vitamin C also may chemically bind metal ions utilized by infectious bacteria that may be roaming around our body; in this way, bacteria cannot access the raw materials they need to grow and multiply. Despite all of these boosts that vitamin C confers on our immune system, you may be surprised to know that the exact biochemical pathways have not yet been described. Calling on what you know about the realities of scientific progress, what factors may have contributed to our gap in knowledge about all of the biochemical pathways of vitamin C? Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 4 of 18 Vitamin C works in the skeletal-muscular, circulatory, nervous, digestive and integumentary (skin) systems as well. In all of these systems, vitamin C is needed for the synthesis of collagen, probably the most prevalent protein in our bodies. If you shop for cosmetics you may have noticed that certain product lines promote the use of vitamin Cbased skin creams and serums. Even in small concentrations, vitamin C promotes collagen production; collagen is often touted for keeping skin “firm” and holding its shape. Cosmetic lines also advocate that vitamin C helps protect the skin because of its antioxidant qualities. Looking at the word parts, and calling on what you already may know from product packaging inserts, what chemical function do you think an antioxidant serves? Vitamin C as Antioxidant One of the more chemically interesting and important functions of vitamin C in the human body lies in its ability to protect the integrity of valuable molecules (e.g., proteins, lipids, carbohydrates, and nucleic acids). Vitamin C helps create chemical blockades against free radicals, atoms or groups of atoms with an odd (unpaired) number of electrons. Antioxidants provide free radicals with the necessary electrons, thereby stopping the free radicals from obtaining them from any available source. Antioxidants can interact safely with free radicals and terminate the chain reaction before vital molecules are damaged. Antioxidants such as ascorbic acid (vitamin C) are commonly added to foods to prevent or delay spoilage due to exposure to free radicals in the air. For example, a cut apple generally browns when exposed to the oxygen in the air. If you submerge cut apple in lemon or orange juice it will not brown, a trick many cooks have used successfully! The ascorbic acid in these juices is serving as an antioxidant. Let’s look more closely at the chemistry behind free radical protection conferred by vitamin C and other antioxidants. Free radicals form under a variety of conditions. Study Figure 2 and list 3 naturally occurring ways in which free radicals form: In the atmosphere, free radicals can be formed when oxygen interacts ozone. Ozone, O3, is a triatomic molecule found in the atmosphere. Its important function is to absorb harmful ultraviolet radiation from space. When the ozone molecule is exposed to ultraviolet radiation in the presence of water (which is almost always available as water vapor), the energy from the ultraviolet light breaks O3 into O2 and two free radicals of OH* (the free radical form of OHˉ). (The asterisk represents an atom in its free radical state.) Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 5 of 18 The free radicals that have been generated are highly reactive and are looking for a source of electrons to allow them to pair up their valence electrons. This makes free radicals very strong oxidizing agents. Oxidizing agents are atoms or groups of atoms that will accept (or even take) electrons from other atoms or groups of atoms. Adapted from the following illustration: http://www.mypowerlab.com/product/benefits/freeradicalattack_humancell.jpg In our ozone example, each of the OH* free radicals is primed to accept one electron from another atom or group of atoms. The atom or group of atoms that provides the electron then becomes positively charged in a process known as oxidation. An atom or group of atoms that loses an electron, and subsequently gains a positive charge, has been oxidized. Another example of an oxidation reaction is the formation of the ionic compound, LiF. In this reaction, the fluorine atom accepts an electron from the lithium atom, thereby oxidizing the lithium atom. This makes the fluorine atom an oxidizing agent, since it causes the oxidation of lithium. Li + F Li+ + F- Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 6 of 18 The Li is oxidized, and therefore becomes more positive (it loses electrons). F facilitated this process by accepting Li’s electron to become reduced, or more negative (it gains electrons). In the box provided below, write the above reaction using Lewis Dot Notation for each element and using arrows to indicate the donation of an electron from lithium to fluorine. Highly reactive free radicals can start a chain of oxidation reactions, producing a domino effect. This may harm important cellular components; cells may die if this occurs. Free radicals are found in the atmosphere and also in toxins and pollutants (e.g., some chemicals, cigarette smoke and illegal drugs) and are formed by normal metabolic processes (See Figure 2). Vitamin C deficiency It is essential to note that even though antioxidants play a crucial role, we do not synthesize them internally; they must be supplied in the diet. Historically, we have seen the symptoms of vitamin C deficiency in a condition called scurvy, which affected many 15th - 18th century sailors on cross-Atlantic journeys. These maritime travelers noticed that they could avoid the symptoms of scurvy—namely fatigue, bruising, bleeding under the skin, and hair and bone loss, all due to the lack of collagen production—if they ate citrus fruits such as oranges and lemons. But it was not until Hungarian researcher Albert Szent-Györgyi isolated and described ascorbic acid (meaning “without scurvy”) that we came to understand the relationship between vitamin C deficiency and scurvy. In 1937, he was awarded The Nobel Prize for Physiology or Medicine for his work with vitamin C. Using the information about your identified sources of naturally occurring vitamin C, as well as any information you have from any supplements or vitamins you take, determine the total amount of vitamin C you ingested yesterday. Based on your data above, did you ingest sufficient vitamin C yesterday to meet the RDA? Explain. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 7 of 18 The Behavior of Vitamin C Vitamin C exists primarily in two forms, ascorbic acid and dehydroascorbic acid. Ascorbic acid readily oxidizes, triggering a three-step oxidation series: ascorbic acid oxidizes to become semi-hydroascorbic acid, which in turn oxidizes to become dehydroascorbic acid (See Figure 3). The propensity for ascorbic acid to oxidize is why much of the vitamin C in foods exists in a partially oxidized form. This oxidation reaction series is reversible; thus, the dehydro- form is still considered to have vitamin activity, as long as it maintains its ability to be reduced back to the ascorbic acid form. A reversible reaction is one in which there is not a complete shift in the reaction equilibrium. Reversible reactions shift back and forth between product and reactant forms in a dynamic equilibrium and are expressed in an equation using a doubleheaded arrow or two half-arrows facing in opposite directions. In this case, the electrons can shift back and forth between ascorbic acid and dehydroascorbic acid via the intermediary form, semi-hydroascorbic acid. On the other hand, dehydroascorbic acid could be further oxidized to form diketogulonic acid. This reaction is irreversible. Think back to Lab #13 and the action of enzymes. Enzymes are made of proteins that denature when exposed to extreme pH or heat. This denaturing of proteins is an irreversible reaction: once the protein denatures, it cannot return to its original form and is now inactive. Similar to the loss of an enzyme’s activity due to its denatured form, once dehydroascorbic acid is oxidized to form diketogulonic acid, its potential vitamin activity is lost. Study Figure 3. Then, in the box below, Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 8 of 18 write the series of chemical equations representing the series of chemical reactions illustrated here. Start with the oxidation of ascorbic acid, and end with the production of diketogulonic acid (3 equations total, including the one provided for you). Remember that when translating a structural formula into a chemical formula you should follow these steps: List the elements in the structure. Choose 1 element and count up the number of atoms. Repeat for all remaining elements. Translate the number of atoms into subscripts. This oxidation process occurs naturally over time. When oranges are still attached to the tree, they are able to continue synthesizing ascorbic acid to replace any that breaks down. This benefits the orange tree because it has a constant source of antioxidants, used to prevent its own free radical damage. Once the orange is picked, however, this ascorbic acid synthesis will cease. However, the ascorbic acid is continually breaking down because it is inherently unstable. Once picked, it also is exposed to air, primarily through the point at which the stem was originally attached, but also through its pores. After a certain period of time and amount of exposure to air, all of the vitamin C in the orange will become diketogulonic acid. This is part of the reason why fresher oranges are more nutritious: they contain the maximum amount of vitamin C. Several abiotic factors can influence the oxidation of ascorbic acid. One of them is temperature. Oranges grow best in sub-tropical climates, where it is almost never below Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 9 of 18 freezing, but it is also not too hot. Florida and California growers depend on this range of temperatures for growing their fruit. Graph the trend describing the relationship between temperature and the production of vitamin C in oranges. Be sure to label your dependent and independent variables: As the temperature increases, reaction rates tend to increase. If we were to heat our orange, do you think this would increase the rate of ascorbic acid oxidation? Conversely, if you keep the orange refrigerated, would this decrease the rate of ascorbic acid oxidation? What does this mean for ensuring that we are actually ingesting the stated RDA of vitamin C per serving size? We will be exploring these ideas in Lab #34. We will be testing refrigerated and heated orange and apple juices for their vitamin C content. We can then determine if heating or cooling has an impact on the amount of vitamin C present in the juices. Materials and Methods Redox Titration Defined In order to determine if temperature affects the amount of available ascorbic acid, we will be using a technique known as a redox titration. This type of titration relies on the same titrating techniques we first used in Lab #27. Interestingly, in order to answer the questions about vitamin C posed above, we will be working with substances that can be oxidized and reduced, instead of working with acids and bases. In order to understand what will be happening in our beakers, we first need to develop an understanding of oxidation and reduction. Even though the ascorbic acid titration does not involve only ionic compounds, it is easier to begin the explanation there. As previously discussed, oxidation is the loss of electrons by an atom or a group of atoms. Oxidation can be represented by a half-reaction. A half-reaction illustrates the behavior of only one of the reactants. The oxidation of magnesium is a very clear-cut half-reaction. Magnesium is a Group 2 element; this means that it readily gives up two Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 10 of 18 electrons to create a more stable state. The half-reaction for the oxidation of magnesium looks like this: Mg Mg2+ + 2eIn the above half-reaction we see magnesium giving up two electrons to form a cation, but where do these electrons go? They will be accepted by another atom or group of atoms. This resulting reaction will be a reduction reaction, where an atom or group of atoms gains electrons, thus becoming reduced, or more negative. This reduction in charge can also be illustrated in a half reaction: O + 2e- O2Notice that oxygen started out electrically neutral, but when it accepted two electrons, it became an anion. The magnesium half-reaction is an oxidation reaction and the oxygen half-reaction is a reduction reaction. We can combine these two half-reactions and obtain an oxidationreduction reaction, or redox reaction. This reaction will illustrate the net movement of electrons in a reaction. For the formation of magnesium oxide, the reaction would be expressed like this: Mg + O Mg2+O2- (or simply MgO) Now that we understand the basics of oxidation and reduction, let’s apply what we know to our lab. We will be using an indicator known as dichloroindophenol (DPIP) to titrate our ascorbic acid. While these larger molecules are characterized as covalent, the same Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 11 of 18 oxidation and reduction behaviors can occur. In class, participate in the How Do We Assign Oxidation Numbers? activity. The Reduction of Ascorbic Acid by DPIP DPIP indicator is an oxidizing agent for ascorbic acid. There is a transfer of two electrons from the ascorbic acid to the DPIP, and thus ascorbic acid is oxidized into dehyrodascorbic acid. When DPIP is in its non-reduced state, it is a salt that, when in solution, has a deep blue color. When it is reduced, DPIP solution becomes colorless (See Figure 4). Thus far, our descriptions of oxidation and reduction have dealt only with ionic compounds in which the movement of ions is very clear-cut. However, the character of a bond might actually be ambiguous. As we saw in Quest I, no bond is purely ionic or purely covalent. We use electronegativity differences to help name bond types, but in nature bonds don’t always fit into our neat little categories How is a given bond most likely to behave based on what we know about it? To answer this question, we can think in terms of criminal profiling. Based on all the data we have gathered, why do we think a particular criminal is behaving as he/she is? Our prediction may not prove true, but we rely on the justice system to weigh all of the evidence. Chemists, too, have constructed a system to help understand and explain the movement of electrons in reactions that are not so clearly ionic. These chemical “suspects,” though not criminals, are identified based on the use of oxidation numbers. An oxidation number is a kind of assumption. It describes the number of charges an atom would have as part of a molecule if we assume that electrons are transferred completely in the direction indicated by the electronegativity difference. Taking hydrogen fluoride, HF, as an example, we see in NYS Physical Setting Chemistry Reference Table S that this compound is classified as polar covalent due to an electronegativity difference of 1.9. The fluorine atom is the more electronegative element, meaning it will more readily accept electrons. The electrons would be transferred from the hydrogen to the fluorine. This is the direction indicated by the electronegativity difference. Hydrogen only has one electron to transfer—so the number of charges in a molecule, assuming electrons are transferred completely, would give hydrogen an oxidation number of +1 and fluorine an oxidation number of -1. Redox reactions can be redefined in terms of oxidation numbers: In a reaction, an element is oxidized if its oxidation number increases, and an element is reduced if its oxidation number is reduced. If this occurs, then the reaction is classified as an oxidation-reduction (redox) reaction. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 12 of 18 The formation of hydrogen fluoride may be described as a redox reaction, since molecular hydrogen (H2) will react with molecular fluorine (F2) to produce two molecules of hydrogen fluoride. Write this reaction in the box below: The oxidation number of H increases from 0 +1; therefore, hydrogen is oxidized. The oxidation number of F decreases from 0 -1; therefore, fluorine is reduced. In case you are confused where the +1 and -1 came from look back up to the paragraph where we discussed using the electronegativity difference to determine oxidation number. It is important to note that ionic charge is indicated using the value of the charge followed by the -/+ sign, whereas oxidation numbers have -/+ sign followed by the value of the oxidation number. There are some basic rules for assigning oxidation numbers, and these can be found in the Assigning Oxidation Numbers handout. Since the titration we are performing involves the transfer of electrons from one substance to another, and therefore a change in oxidation numbers, it fits into the class of titrations called redox titrations. They differ from acid-base titrations, such as the one we performed in Lab #27, because redox titrations involve the transfer of electrons, and acidbase titrations involve the transfer of protons and generally result in neutralization. We will be adding drops of DPIP from the burette into a flask containing a solution of ascorbic acid with a known concentration. In general, when performing a titration, you add drops until the endpoint is reached. What is the endpoint of a titration? What are some endpoints you have seen in previous titrations? Record your thoughts in the space provided. In our case, we are adding blue DPIP to clear ascorbic acid. The DPIP turns colorless when it reacts with the ascorbic acid. Therefore, when we have drops that do not turn colorless, but instead remain blue, we know that all of the ascorbic acid has reacted, and thus the DPIP does not get reduced, and we have reached our endpoint. Alas, it is not quite that simple with ascorbic acid. Remember that the oxidation of ascorbic acid to dehydroascorbic acid is reversible. So, as we convert to dehydroascorbic acid, some of it simultaneously converts back to ascorbic acid. This makes obtaining exact volumes a little difficult. To reduce this source of error, we will be mixing our ascorbic acid with oxalic acid. Oxalic acid, C2H2O4, is a naturally-occurring compound Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 13 of 18 found in many food items. It is composed of two linked carboxyl groups, (carbon doubled bonded to an -oxygen, and single bonded to an –OH). Write a structural formal and the empirical formula for oxalic acid in the space below. (Hint: refer to Lab #33 or your NYS PS Chemistry Core Curriculum Guide if you need a refresher on empirical formulas.) Oxalic acid is one of the simplest of the organic acids (See NYSPS Reference Table R). It is fatal to humans at about 1500 mg. Even though we consume it every day in foods such as spinach, we also eliminate much of it in our urine. Oxalic acid reacts with the dehydroascorbic acid produced by the reaction with DPIP to produce diketogulonic acid (Figure 3). This means that the dehydroascorbic acid will not reverse to its ascorbic acid form and that the DPIP that was reduced in the reaction will remain colorless. Therefore, before we titrate our ascorbic acid, we will be mixing it with some oxalic acid to prevent the reversal of our oxidation reaction. Then we can proceed with our titration to our endpoint, which is observed as a rose-pink (or even amber-colored) solution. Determination of Concentration Through the Use of a Standard Curve In Lab #27 we used stoichiometric calculations to determine concentration. Alternatively, in Lab #32 we used qualitative data to create a standard curve that described concentration. In this lab we will be applying the technique of using a standard curve using known quantitative concentrations of ascorbic acid, and this will allow us to obtain much more accurate results than the relative brightness observations we used in Lab #32. By titrating several different solutions of known concentration, we will be able to construct a curve of Concentration of Ascorbic Acid vs. Volume of DPIP Used. This is standard operating procedure for many experiments that attempt to determine the concentration of an unknown. Once we have a standard curve, we can titrate solutions of unknown concentration of ascorbic acid and see how many milliliters of DPIP are necessary to convert all of the ascorbic acid to dehydroascorbic acid. By plotting these volumes, we can interpolate the concentration of ascorbic acid in the samples just as we did with relative brightness in Lab #32. Ultimately, we will be experimentally determining how the heating of juices affects the amount of vitamin C present. Our unknowns will be samples of orange and apple juice that have been heated. The fact that we are using fruit juices will affect our ability to determine the endpoint. Why is this true? Record your thoughts. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 14 of 18 How might we prepare ourselves for the variation in endpoint color? Write down some ideas. Materials Per Team of 2: goggles Preparation of Stock Ascorbic Acid Solution 1 piece filter or weigh paper balance ascorbic acid 10 ml graduated cylinder 100 ml beaker distilled water Parafilm® Preparation of Dilutions/ Known Mass of Ascorbic Acid Solutions 100 ml Erlenmeyer flask 1 pipette bulb 20 ml graduated pipette oxalic acid NOTE* - This is a corrosive acid, so avoid contact with skin and eyes. If contact occurs, flush with water immediately. 2 - 5 ml graduated pipettes 1 M HCl NOTE* - This is a corrosive acid avoid, so avoid contact with skin and eyes. If contact occurs, flush with water immediately. stock solution of ascorbic acid Titration of Solution of Known and Unknown Mass of Ascorbic Acid 25 or 50 ml buret burette or ring stand burette clamp 100 ml Erlenmeyer flask 50 ml of 2 - 6 dichloroindophenol (DPIP) NOTE* This is an oxidizing indicator. Avoid contact with skin and eyes. If contact occurs, flush skin with cold water. Sample of one of the following: refrigerated orange juice heated orange juice refrigerated apple juice heated apple juice apple juice exposed to a basic solution Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 15 of 18 fluid surrounding canned vegetable Construction of Standard Curve for Mass of Ascorbic Acid Class Data Table: Solutions of Known Mass of Ascorbic Acid Class Graph: Mass of Ascorbic Acid vs. Volume of DPIP Class Data Table: Solutions of Unknown Mass of Ascorbic Acid Procedure: Preparation of Stock 0.25 M Ascorbic Acid Solution *Note if you have 10 mL volumetric flasks, you can substitute those for graduated cylinders and thus improve your accuracy in solution preparation. 1. Obtain materials. 2. Fold filter paper in half, and reopen. 3. Place on balance and tare. 4. Mass out 0.44 g of ascorbic acid on reopened filter paper. 5. Using the center of the fold as a pouring guide, pour ascorbic acid into the graduated cylinder; gently tap back side of filter paper to loosen all grains. 6. Using a dropper or Pasteur pipette, dilute with distilled water up to about 7 ml. 7. Seal the top of the graduated cylinder with Parafilm®. 8. Invert carefully to mix solution. 9. Turn right side up and remove Parafilm®. Fill to a volume of 10 ml. 10. Recover with Parafilm® and invert again to mix. 11. Transfer to labeled stock container. 12. Use immediately, or refrigerate until used. 13. Clean up and wash hands. Preparation of Dilutions/ Known Mass of Ascorbic Acid Solutions 14. Obtain materials. 15. Label the 100 ml Erlenmeyer flask with appropriate grams of ascorbic acid (see Pre-Lab #13). 16. Using a graduated pipette and bulb, transfer 18 ml of oxalic acid to the 100 ml Erlenmeyer flask. 17. Using a second pipette, transfer 2 ml of 1 M HCl to the flask. 18. Gently swirl. 19. Using a third pipette, possibly one already in the stock solution, transfer 1, 2, 3 or 4 ml of ascorbic acid solution to the flask. Take care not to touch the pipette to the sides of the flask, or else you will contaminate the stock. Note*--end solutions will be of volumes ranging from 21 mL – 24 mL. Be sure to record the volume of your solution. 20. Gently swirl. Preparation of Dilutions of Unknown Mass of Ascorbic Acid Solutions 21. Obtain materials. 22. Label the 100 ml Erlenmeyer flask with the name of the unknown solution. 23. Using a graduated pipette and bulb, transfer 18 ml of oxalic acid to the 100 ml Erlenmeyer flask. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 16 of 18 24. Using a second pipette, transfer 2 ml of 1 M HCl to the flask. 25. Gently swirl. 26. Using a third pipette, possibly one already in the stock solution, transfer 2 ml of ascorbic acid solution to the flask. Take care to not touch the pipette to the sides of the flask, or else you will contaminate the stock. Be sure to record the volume of your solution. 27. Gently swirl. Titration of Solution of Known and Unknown Mass of Ascorbic Acid 28. Obtain materials. 29. Rinse the burette with 1-2 ml of DPIP. Pour waste into the appropriate waste container. 30. BE SURE THE STOPCOCK IS CLOSED. 31. Fill the burette and record the starting volume of your team’s known ascorbic acid solution concentration. 32. You may wish to get a feel for your burette’s stopcock before commencing with the titration. Perform a few practice drops into a waste container. *Note: If you do practice making drops, be sure to record your new starting volume. Titrate your known ascorbic acid solution, one drop every five seconds, until an endpoint of a rose pink color persists for about 5 seconds. 33. Record the final volume of known ascorbic acid in the burette. 34. DO NOT DRAIN THE BURETTE. 35. You’re not starting from scratch, so the directions should reflect that through #33. Obtain ____ ml of your unknown. (see Pre-Lab # s 11, 12) 36. Label the 100 ml Erlenmeyer flask with the name of the unknown solution. 37. Using a graduated pipette and bulb, transfer 18 ml of oxalic acid to the 100 ml Erlenmyer flask. 38. Using a second pipette, transfer 2 ml of 1 M HCl to the flask. 39. Gently swirl. 40. Add the volume of your unknown solution (Step 35) to the flask and gently swirl. 41. Record the starting volume of DPIP for the unknown. You haven’t drained the burette, so give directions for what the starting volume of DPIP will be. 42. Titrate the unknown solution one drop every five seconds until you reach the end point. *Note: If you are using a colored starting solution, do you expect the endpoint color to be a little lighter or darker? For example, if an endpoint is supposed to be red, and you are titrating a blue solution, then your endpoint will be blue + red which is purple. 43. Record the final volume DPIP for the unknown solution. 44. Dispose of all wastes as indicated by your instructor. 45. Clean all glassware. 46. Wash hands. Construction of a Standard Curve for the Mass of Ascorbic Acid 47. Calculate and record the total volume of DPIP used for your known. 48. Transfer this value to the Class Data Table and Graph. 49. Calculate and record the total volume of DPIP used for your unknown. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 17 of 18 50. Transfer this value to the Class Data Table. 51. Record all Class Data in your own lab notebook. Post – Lab Activities: 1. Based on the Class Data Tables, construct the Class Standard Curve in your lab notebook and then plot the volumes of all the unknown samples and interpolate their masses. Record these values. 2. Calculate the molarities of the unknown solutions based on their masses. Remember, molarity is based on the volume of solution. Record these values. 3. As a lab group, reflect on your hypothesis. Did the data support it? Why or why not? 4. Class Debrief 5. Based on your data, do you think that cooking food impacts the Vitamin C content? 6. Respond to FNF II Food, Nutrition and Fitness Strand Map: Dietary Supplements Question #4. Lab #34: Investigating the Effects of Heat on Ascorbic Acid ©2002-2006 EduChange® 18 of 18