Chem Activity Potential Energy
advertisement

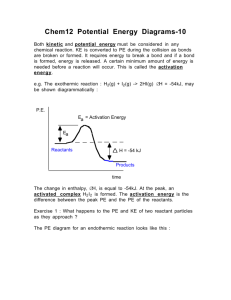
Chem Activity: Potential Energy Information: The system is the region that you are focusing on while the surroundings include the space around the system. When a chemical reaction takes place, the reactants and products are considered the system, while the container and air or other materials that may come in thermal contact are considered the surroundings. Heat travels from areas of high heat to low heat. Endothermic processes occur when heat is absorbed from the surroundings into the system. Exothermic processes occur when heat is released into the surroundings from the system. All chemical reactions are accompanied by a change in heat content. Reactions that absorb energy would be considered endothermic. Reactions that release energy would be considered exothermic. Enthalpy (H) is the amount of heat content that a substance has at a given temperature and pressure. During a chemical reaction, the heat content of the substances changes during the progression of the reaction. A reaction profile is a graphical representation of what is happening to the enthalpy (heat) of a reaction. The potential energy (PE) of the reactants and products are shown on the graph. The activation energy (Ea) is the energy needed to start the reaction. The transition state (TS) or the activated complex is the brief interval of bond disruption (reactants) and bond formation (products). Catalysts increase the rate of reaction without being used up in the reaction. The models used in this activity are an Excel file courtesy of Scott Sinex at Prince George Community College. Hover on any cell that has a red flag in the corner for some important tips. VBCPS 2011 Created by Heather Green and Bridget Mariano Select the PE Diagram tab from the bottom navigation bar. Model 1: Potential Energy Diagram Questions: 1. What is the potential energy of the reactants? products? 2. What is the potential energy of the transition state or the activated complex? 3. Click on the green “Activation energy” box. Record the value of the activation energy (Ea) for this reaction. 4. How is the activation energy calculated? Write the equation. 5. Is the value of the Ea positive or negative? What are the units? 6. Unclick the green box and click on the brown “Add a catalyst” box. What happens to the activation energy when a catalyst is added? 7. How does the addition of a catalyst affect the potential energies of the reactants and the products? 8. Would a catalyst speed up or slow down the rate of reaction? 9. Click on the teal “Activation energy with a catalyst” box (keep brown box checked). Determine the Ea for the reaction upon the addition of a catalyst. Show your work. 10. Is the value of the Ea positive or negative? 11. Unclick both the brown and teal boxes. Click on the purple “Heat of Rxn” box. Record the value for the Change of the Enthalpy/Heat of Reaction (H) for this reaction. Include the sign and the units for the value. 12. How is the heat of reaction (H) calculated? Write the equation. 13. Is the value of the H positive or negative for this graph? 14. Is energy absorbed or released by this reaction? 15. Is the reaction represented by this graph endothermic or exothermic? 16. Write a general statement concerning the value of the H and the classification of the reaction as endothermic or exothermic. Sketch and label a sample of this reaction profile on the last page of this activity. VBCPS 2011 Created by Heather Green and Bridget Mariano Uncheck all of the boxes. Use these buttons to manipulate the reaction profile graph. reactants activated complex products Model 2: Manipulated Potential Energy Diagram Manipulate the graph so that the potential energy of the reactants is +30 kJ, the potential energy of the products is +10 kJ and the potential energy of the activation energy is +40 kJ. Questions: 17. What is the potential energy of the reactants? products? 18. What is the potential energy of the transition state or the activated complex? 19. Click on the green “Activation energy” box. Record the value of the activation energy (Ea) for this reaction. 20. Calculate the activation energy (show all work). 21. Is the value of the Ea positive or negative? What are the units? 22. Unclick the green box and click on the brown “Add a catalyst” box. What happens to the activation energy when a catalyst is added? 23. How does the addition of a catalyst affect the potential energies of the reactants and the products? 24. Click on the teal “Activation energy with a catalyst” box (keep the brown box clicked). Determine the Ea for the reaction upon the addition of a catalyst. Show your work. 25. Is the value of the Ea positive or negative? 26. Unclick both the brown and teal boxes. Click on the purple “Heat of Rxn” box. Record the value of the Change of the Enthalpy/Heat of Reaction (H) for this reaction. Include the sign and the units for the value. 27. Calculate the heat of reaction (H) – show all work. 28. Re-click the brown box. Does the H change for the reaction? Does your observation match your answer to question 23? 29. Is the value of the H positive or negative for this graph? VBCPS 2011 Created by Heather Green and Bridget Mariano 30. Is energy absorbed or released by this reaction? 31. Is the reaction represented by this graph endothermic or exothermic? 32. Write a general statement concerning the value of the H and the classification of the reaction as endothermic or exothermic. Sketch and label a sample of this reaction profile on the last page of this activity. Extension activity Complete the assigned worksheets on reaction profiles. VBCPS 2011 Created by Heather Green and Bridget Mariano