Matter Test Review Sheet
advertisement
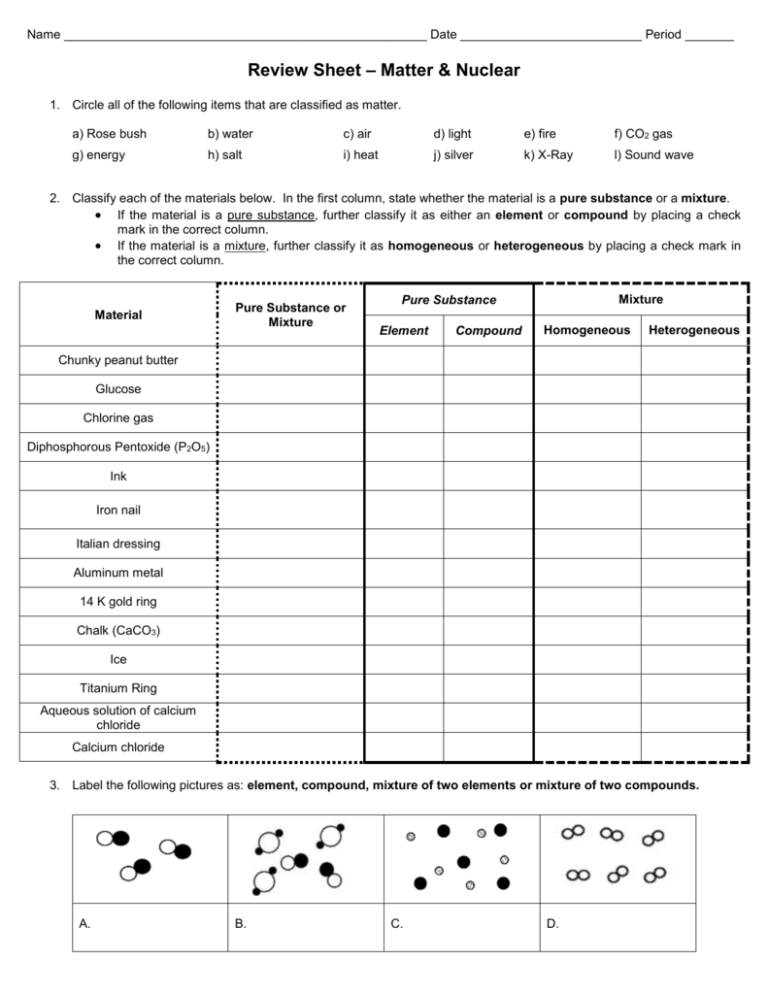
Name ____________________________________________________ Date __________________________ Period _______ Review Sheet – Matter & Nuclear 1. Circle all of the following items that are classified as matter. a) Rose bush b) water c) air d) light e) fire f) CO2 gas g) energy h) salt i) heat j) silver k) X-Ray l) Sound wave 2. Classify each of the materials below. In the first column, state whether the material is a pure substance or a mixture. If the material is a pure substance, further classify it as either an element or compound by placing a check mark in the correct column. If the material is a mixture, further classify it as homogeneous or heterogeneous by placing a check mark in the correct column. Material Pure Substance or Mixture Mixture Pure Substance Element Compound Homogeneous Heterogeneous Chunky peanut butter Glucose Chlorine gas Diphosphorous Pentoxide (P2O5) Ink Iron nail Italian dressing Aluminum metal 14 K gold ring Chalk (CaCO3) Ice Titanium Ring Aqueous solution of calcium chloride Calcium chloride 3. Label the following pictures as: element, compound, mixture of two elements or mixture of two compounds. A. B. C. D. 4. Place an X in each box that describes the property. Chemical Property Physical Property INTENSIVE Physical Property EXTENSIVE The tendency of ethyl alcohol to burn Silver is a shiny metal The temperature at which dry ice evaporates The volume of methanol is 39.6 mL Chlorophyll, a plant pigment, is green The flammability of propane gas A 14.5 g sample of iron The density of titanium metal is 4.5 g/cm 3 Hydrogen gas does not conduct electricity H2 gas reacts vigorously with O2 to form H2O 5. Put an X in the box that best describes each of the following substances. Physical Change Iron in rock combining with oxygen to form hematite Carbonic acid weathering limestone Freezing water cracking limestone Flowing water eroding a limestone riverbed Acid rain forming puddles on limestone Coastal waves dissolving limestone sediments Chemical Change Physical Change Combustion of gasoline Water boiling Breaking Glass Grilling a piece of chicken Iodine subliming Reacting sodium with chlorine Biting a pear A fungus decomposing wood Digesting a pear Painting Wood 6. What are the 4 indicators that a chemical reaction has occurred? 1) 2) 3) 4) 7. Fill in the table below. 3 basic Physical states of matter Definite shape? Yes or No Definite volume? Yes or No Circle the example of each Water vapor/Steam Ice Water Water vapor/Steam Ice Water Water vapor/Steam Ice Water Chemical Change 8. Answer the following questions about the particle diagrams below a) What type of matter is represented in the before picture? ____________________ b) What type of matter is represented in the after picture? ____________________ c) What state(s) of matter is/are represented in the before picture? ____________________ d) What state(s) of matter is/are represented in the after picture? ____________________ e) What kind of change occurred? ______________________ 9. For each of the following particle diagrams decide what type of matter is present (pure element, pure compound, mixture of elements, mixture of compounds, or mixture of elements and compounds) and what state/phase of matter the substance is in Type of matter: _____________ Type of matter: _____________ Type of matter: _____________ State of matter: _____________ State of matter: _____________ State of matter: _____________ 10. Use the phase diagram to answer the following questions. What is the phase of water at 2 atm and 50oC? _______________ What phase change will occur if the temperature is lowered from 80oC to -5oC at 1 atm? _______________ You have ice at -10oC and 1 atm. What could you do in order to cause the ice to sublime? _____________________________________________ Draw an arrow representing condensation on the diagram. 11. Use the heating curve to answer the following questions. Label the solid, liquid and gas regions on the heating curve. What is the melting point (in oC) of this substance? What is the boiling point (in oC) of this substance? As energy is added from point A to point B, what phase change is occurring? As energy is added from point C to point D, what phase change is occurring? The phase changes from point A to point B and from point C to point D are both _______________ processes? As energy is released from point D to point C, what phase change is occurring? As energy is released from point B to point A, what phase change is occurring? The phase changes from point D to point C and from point B to point A are both _______________ processes? 12. Complete the chart: Type of Radiation/Particle Beta Material needed to stop/shield radiation Isotopic Notation Alpha Gamma 13. Indicate the symbol, the mass number and the atomic number of the missing reactant or product in each of the following nuclear reactions. a. 95 Nb 95Mo ______ 41 42 c. b. 22 P 0e _______ 15 1 d. 14. Given the equation: 6 C 7 N 14 14 _______ 206Pb 4He 82 2 42Ti ______ 42Sc 22 21 X , what is the name of the particle is represented by the letter X? __________ 15. The decay series of uranium-235 is α, α, α, α, β, β, α. Write an equation for each step in this decay series.







