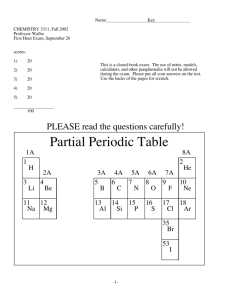
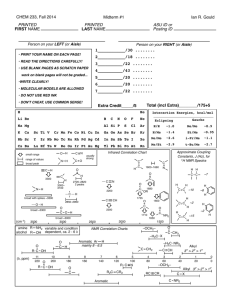
CHE 252
advertisement

CHE 252 Laboratory: Sodium Borohydride Reduction of Benzophenone Spring 2011 Reminder: This lab is tentatively worth 18 points. Your lab report’s results section should include all TLCs (scaled reproductions of your actual TLCs drawn into the results section) with their Rf values. Introduction: 2 pts. Results: TLC slides reproduced in the results section Table of Rf values Melting point Percent yield calculation Sample appearance Labeled 1H and 13C NMR spectra of product and starting material Discussion: Writing/Format Questions 1 pt. 1 pt. 1 pt. 2 pts. 1 pt. 2 pts. 3 pts. 2 pts. 3 pts. Questions: 1. (1 pt) If the rate of heating is too high while heating the reaction mixture under reflux, what will happen to the solvent? 2. (2pts) In the reduction of an acyclic aldehyde or ketone (e.g. benzophenone) the hydride ion can attack from either side of the planar carbonyl unit with equal probability. When 4-tert-butylcyclohexanone (a monocyclic ketone) reacts with NaBH4 in methanol two alcohol stereoisomers are possible. The reaction seems to be influenced by thermodynamic factors. Draw the structures of the two stereoisomers and circle the isomer that will be in the greater amount due to being thermodynamically more stable.