(04.10.2010\) Maria Griffiths - Boiling-Point
advertisement
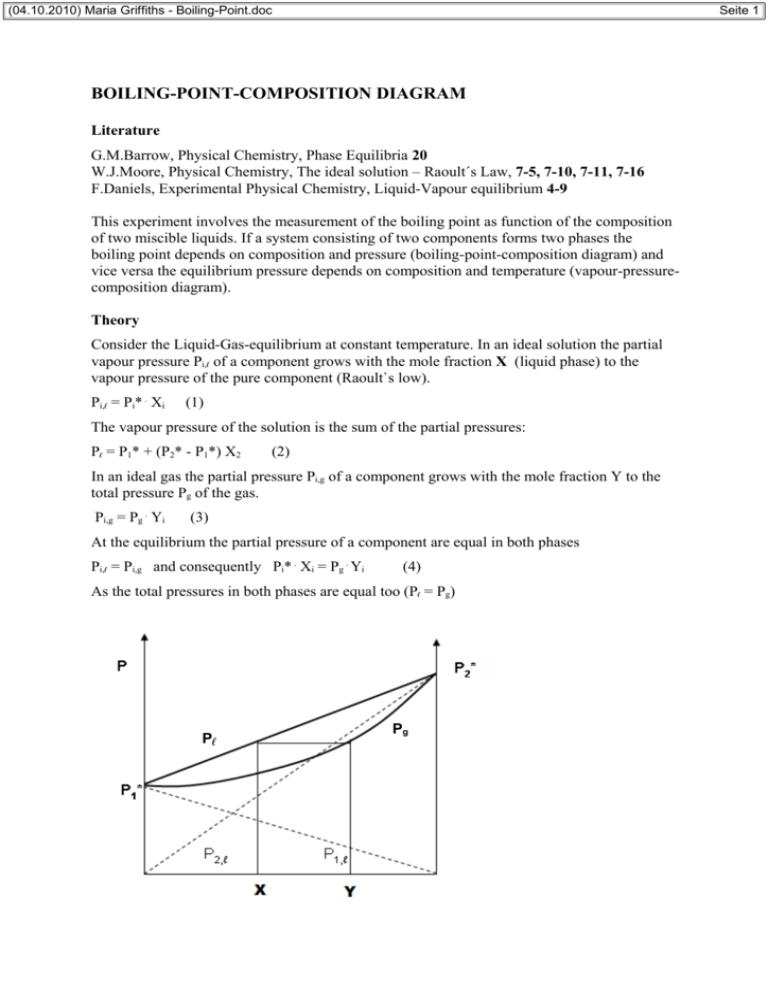
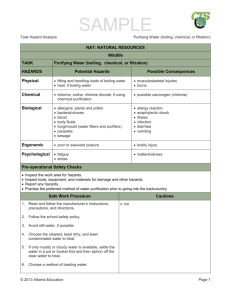
(04.10.2010) Maria Griffiths - Boiling-Point.doc Seite 1 BOILING-POINT-COMPOSITION DIAGRAM Literature G.M.Barrow, Physical Chemistry, Phase Equilibria 20 W.J.Moore, Physical Chemistry, The ideal solution – Raoult´s Law, 7-5, 7-10, 7-11, 7-16 F.Daniels, Experimental Physical Chemistry, Liquid-Vapour equilibrium 4-9 This experiment involves the measurement of the boiling point as function of the composition of two miscible liquids. If a system consisting of two components forms two phases the boiling point depends on composition and pressure (boiling-point-composition diagram) and vice versa the equilibrium pressure depends on composition and temperature (vapour-pressurecomposition diagram). Theory Consider the Liquid-Gas-equilibrium at constant temperature. In an ideal solution the partial vapour pressure Pi,ℓ of a component grows with the mole fraction X (liquid phase) to the vapour pressure of the pure component (Raoult`s low). Pi,ℓ = Pi* . Xi (1) The vapour pressure of the solution is the sum of the partial pressures: Pℓ = P1* + (P2* - P1*) X2 (2) In an ideal gas the partial pressure Pi,g of a component grows with the mole fraction Y to the total pressure Pg of the gas. Pi,g = Pg . Yi (3) At the equilibrium the partial pressure of a component are equal in both phases Pi,ℓ = Pi,g and consequently Pi* . Xi = Pg . Yi (4) As the total pressures in both phases are equal too (Pℓ = Pg) Pℓ Pg (04.10.2010) Maria Griffiths - Boiling-Point.doc Seite 2 Vapour-Pressure-Composition Diagram (T = const.) Y2 is related to X2, and consequently Pg is a function of Y2. Pg = P1* + (P2* - P1*) Y2 . Pg/ P2* (5) Is the pressure kept constant (P0 = const. = Pℓ = Pg) the equilibrium temperature (boiling point) T is a function of X (and of Y also). In an ideal solution we know the vapour pressure as P0 = P1*(T) . X1 + P2*(T) . X2 (6) But we have to consider that the pressures Pi* of the pure components depend on temperature. As for ideal solutions the boiling points T(X) are located between the boiling points of the pure components (T1, T2) for all these points the corresponding composition X can be calculated with the aid of a proper Function P*(T). For moderate pressures ClausiusClapeyron is applied. The pertinent composition in the gas phase (also at the temp. T) is calculated according eq. (4). Pi*(T) . Xi = P0 . Yi (4a) T T1 Tg Tℓ T2 X Y Boiling-Point-Composition Diagram (P = const.) Eq. (1) is the ideal form of the real eq. valid for nonideal solutions. Pi,ℓ = Pi* . a2 = Pi* . γi . Xi (7) a, γ activity, activity coefficient This eq. is applied on solutions forming an azeotrope composition. For such a solution eq. (6) is modified (with eq. (7)) to: P0 = P1*(T) . γ1 . X1 + P2*(T) . γ2 . X2 (8) The activity coefficient depends on the composition. Van Laar proposes for nonelectrolytes: log γ1 = A1/[1 + (A1X1/A2X2)]2 log γ2 = A2/[1 + (A2X2/A1X1)]2 (9) The Van Laar coefficients depend on both the pressure and the temperature. But the influence of pressure is negligible und that one of temperature is smaller than a few percent at ∆T < 20 o C. At moderate pressures the ideal gas equation describes the gas phase of an azeotropic system realistically enough. Thus eq. (4a) reads (04.10.2010) Maria Griffiths - Boiling-Point.doc Pi*(T) . γi . Xi = P0 . Yi (10) The Van Laar coefficients Ai are calculated using composition X and boiling point Taz of the azeotrope (where X = Y) in eq. (10). Pi*(Taz) . γi,az = P0 (11) If in eq. (8) the activity coefficients γi are replaced by the Van Laar coefficients Ai a definite correlation between boiling point T and composition X is obtained. Unfortunately nether the function T(X) nor the function X(T) can be calculated in a direct way (because of the exponential). In the homepage of the Institute of Polymer Science you will find a program for the calculation of the boiling-point-composition diagram based on the eqs. shown above where Newton´s approximate method is used for T(X) Experiment Show the boiling-point-composition diagram T(X,Y) of a binary system at atmospheric pressure (P0) by studying the balance between evaporation and condensation in different mixtures. The concentrations (Vol %) will be determined by the refraction index n. 1. Measurement of the calibration curve: Prepare 5ml each of a 20, 40, 60 and 80% mixture volumetrically. To analyse it, it is enough to: Vol% B = VB(VA + VB) although VA + VB ≈ VA+B The refraction index of this mixture as well as the pure component is determined in an Abberefractometer at 20° C exact. 2. Measurement of the boiling-point-composition diagram: 40 ml of the pure component or the mixtures have to be brought to a boil while using the backflow condenser and the constant boiling point is defined. Use the heating mantle just enough to keep it at a moderate boil. Tb is measured at the transition liquid to steam. The thermocouple should be immersed in steam bubbles. The mixtures are made in dilution series of up to 50 %, while the volume mass (40 ml) should stay about the same. Meaning before adding the 2nd component you have to take out the same amount first. Which component you start with and what mixture has to be made will be given. Cool before taking out residue and condensate and measuring the refraction index of them. Taking of sample and measuring of refraction index have to be done quickly as evaporation might change the concentration. Only use a plastic spatula to spread the sample on the glass prism of the refractometer. Tables: Calibration (n, Vol%), Boiling-Point-Composition (Tb, n ℓ, % ℓ, ng, %g) Graphs: Calibration curve Vol %(n) with regression analyses Boiling-point-composition diagram Tb(% ℓ and %g) Equilibrium diagram %g(% ℓ) for determination of the azeotropic point 3. Calculation of the boiling-point-composition diagram: After the measurements where taken approved, the substances will be given. Please download the program from the homepage of the Institute of Polymer Science. Use the measured data – boiling points, azeotropic concentration, atmospheric pressure. Show the result as table and as graph. In the calculated diagram the measured diagram is drawn (table it Tb, % ℓ, X, %g, Y, and cite the eq. for conversion of Vol% in X). Seite 3