Lab 4 - Dr. Chad Landrie
advertisement

CHEM 233: Organic
Laboratory I Prelab Lecture
University of Illinois
at Chicago
UIC
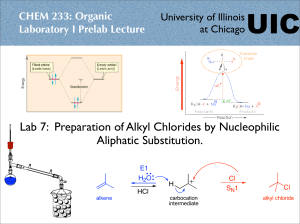
Lab 4: Separation of Ethyl Acetate and Butyl Acetate by
Fractional Distillation. Analysis of fractions by Gas-Liquid
Chromatography.
Simple Distillation: Applications & Goals
Applications:
Today’s Goals:
1. Separate mixtures of
volatile liquids not separable
by simple dist. (∆bp < 50 ºC)
1. Separate a 1:1 mixture of
ethyl acetate and butyl
acetate by simple distillation.
2. Separate hydrocarbons in
crude oil with fractionating
tower (refining).
O
H3C
O
O
CH3
Ethyl Acetate
H3C
O
CH3
Butyl Acetate
2. Compare GLC results
from fractional to simple.
University of
Illinois at Chicago
UIC
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 2
Prelab Lecture: Lab 4
Fractional Distillation Apparatus*
Notes:
thermometer
adapter
{
stillhead
vacuum adapter
(used here as a driptip)
water out
(to drain)
pipette
bulb
water in
(from faucet)
West
Condenser
Hempel
Column
2. Pipette bulbs = seal air inside outer jacket of
Hempel column = insulated = uniform
temperature gradient.
3. Raschig rings = increase surface area =
increase number of theoretical plates = better
separation.
4. Copper wire plug may be necessary for some
Hempel columns to prevent Raschig rings from
falling in stillpot.
Raschig
rings
stillpot
(round bottom flask)
1. See notes for Lab 3 setup.
5. Use 1-2 boiling stones in stillpot.
distillate
6. Stillpot should not be larger than 100 mL.
* Locations of Bunsen clamps are not shown.
University of
Illinois at Chicago
UIC
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 3
Prelab Lecture: Lab 4
Temperature Gradient Required for Fractionation
Process:
Temperature Gradient
Lower Temp
(T = bp of component
currently distilling)
Higher Temp
(T = bp mixture
in stillpot)
1. Temperature gradient is usually established
automatically so long as column is insulated.
2. Many vaporizations and condensations take
place inside Hempel column.
4. The vapor phase becomes more and more
concentrated in the more volatile component
with each vaporization/condensation cycle since
the more volatile component has higher Pº and
since the Nx increases with each cycle.
(P = Pº * Nx; Raoult’s Law)
5. Vaporization/condensation cycle = theoretical
plate. More theoretical plates = better
separation.
University of
Illinois at Chicago
UIC
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 4
Prelab Lecture: Lab 4
Temperature-Composition Diagram
Component A
more volatile
higher Eq. Vapor Pressure (P°)
lower boiling point
vapor line: calculated
using Raoult's Law
98
2
90 10
77 33
50 50
33 77
%A
%B
10 90
UIC
liquid line: boiling point
of the liquid mixture
2 98
University of
Illinois at Chicago
Each “step” =
one theoretical plate
b.p. of A
Vaporization
liquid to vapor
> P° = more vaporization
composition changes
Raoutl's Law: P=Na*P°
b.p. of B
Condensation
vapor to liquid
composition does not change
surface of column or packing
Temperature
Component B
less volatile
lower Eq. Vapor Pressure (P°)
higher boiling point
% Composition
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 5
Prelab Lecture: Lab 4
Theoretical Plates
Factors Affecting Theoretical
Plates (N)
HETP = height equivalent to a
theoretical plate
1. temperature gradient
2. surface area (↑SA=↑N)
3. column length (↑L=↑N)
4. rate of gas flow (GC only)
• measure of the efficiency of a
fractionating column or GC column
• lower HETP = less height needed for
1 N = more efficient column
HETP =
University of
Illinois at Chicago
UIC
column height or length (L)
theoretical plates (N)
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 6
Prelab Lecture: Lab 4
Collect Three Fractions
Construct a graph similar* to the one below in your
course manual according to the instructions.
Temperature (ºC)
Fraction 2
(dramatic change in
temperature)
Fraction 3
(relatively constant
temperature)
Fraction 1
(relatively constant
temperature)
Volume of Distillate (mL)
30
*If your graph does not look like this, your thermometer may be misplaced or you may be distilling faster than 1-2 drops/second.
University of
Illinois at Chicago
UIC
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
Slide 7
Prelab Lecture: Lab 4
Comparison of V (distillate) vs. T Graphs
Temperature (ºC)
Temperature (ºC)
Which graph below represents the most
efficient separation of a binary mixture? Why?
Which most likely represents simple distillation
and which fractional distillation? Why?
Volume of Distillate (mL)
University of
Illinois at Chicago
UIC
30
Volume of Distillate (mL)
© 2009, Dr. Chad L. Landrie
CHEM 233: Organic Chemistry Laboratory 1
30
Slide 8
Prelab Lecture: Lab 4