hw05soln - WordPress.com
advertisement

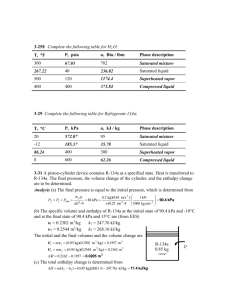
Name: HW #05 Due: 03/06/2012 ES 346 Solution Set 4-7 1 of 12 Problem: A piston–cylinder device initially contains 0.07 m3 of nitrogen gas at 130 kPa and 120°C. The nitrogen is now expanded polytropically to a state of 100 kPa and 100°C. Determine the boundary work done during this process. Solution: From Tbl A-2: R = 0.2968 kJ kg ⋅ K Assuming the nitrogen is an Ideal Gas, determine the mass of the gas in the system and the volume of the system after the expansion (state 2): (130 kN m )(0.07 m ) kNkJ⋅ m N2 130 kPa 120°C 3 2 m= P1V1 = = 0.07802 kg RT1 0.2968 kJ (120 + 273 K) kg ⋅ K mRT2 = V2 = P2 3 (100 + 273 K) (0.07802 kg) 0.2968 kPa ⋅ m kg ⋅ K = 0.08637 m 3 100 kPa For a polytropic process: P1V1n = P2V 2n ⇒ (130 kPa)(0.07 m 3 ) n = (100 kPa)(0.08637 m 3 ) n ⇒ n = 1.249 The boundary work for a polytropic process is determined as: P2V 2 − P1V1 1− n (100 kPa)(0.08637 m 3 ) − (130 kPa)(0.07 m 3 ) kJ = = 1.86kJ 3 1 − 1.249 kPa ⋅ m Wb = Ans hw05soln.docx HW #05 Due: 03/06/2012 Name: Solution Set ES 346 4-10 2 of 12 Problem: A mass of 5 kg of saturated water vapor at 300 kPa is heated at constant pressure until the temperature reaches 200°C. Calculate the work done by the steam during this process. Solution: Assume that the process is quasi-equilibrium. Note that the pressure remains constant during this process. We can use Tbl A-4 thru A-6 to obtain the specific volumes at the initial and the final states. We can also plot this information on a P-v Process Diagram as shown: P1 = 300 kPa 3 ⇒ v 1 = v g = 0.60582 m /kg Sat. vapor P (kPa) 300 1 2 P2 = 300 kPa 3 ⇒v 2 = 0.71643 m /kg T2 = 200°C V From its definition, the boundary work is determined to be: 2 Wb ,out = ∫ P dV = P (V 2 − V1 ) = mP (v 2 − v 1 ) 1 1 kJ = (5 kg)(300 kPa)(0.71643 − 0.60582) m 3 /kg 3 1 kPa ⋅ m = 165.78kJ Ans The positive sign indicates that work is done by the system (work output). hw05soln.docx Name: HW #05 Due: 03/06/2012 Solution Set ES 346 4-25 3 of 12 Problem: 1-kg of water that is initially at 90°C with a quality of 10 percent occupies a spring-loaded piston–cylinder device, such as that in Fig. P4–25. This device is now heated until the pressure rises to 800 kPa and the temperature is 250°C. Determine the total work done during the process in kJ. Solution: P Assume that the process is in quasi-equilibrium. 2 800 kPa The initial state is given to be a saturated mixture at 90°C. 1 From Tbl A-4: P1 = 70.183 kPa v v 1 = v f + x(v g − v f ) = 0.001036 m = 0.23686 m 3 kg + (0.10)(2.3593 − 0.001036) m 3 kg 3 kg At the final state conditions of 800 kPa and 250°C, from Tbl A-6: 3 v 2 = 0.29321 m kg The work done is equal to the area under the process line 1-2: P1 + P2 m(v 2 − v 1 ) 2 (70.183 + 800)kPa 1 kJ = (1 kg)(0.29321 − 0.23686)m 3 3 2 1 kPa ⋅ m = 24.52kJ Wb ,out = Area = Ans hw05soln.docx Name: HW #05 Due: 03/06/2012 4-34 4 of 12 Solution Set ES 346 Problem: Saturated water vapor at 200°C is isothermally condensed to a saturated liquid in a piston–cylinder device. Calculate the heat transfer and the work done during this process, in kJ/kg. Solution: Assume that the cylinder is stationary and thus the kinetic and potential energy changes are zero. Also assume that there are no work interactions involved other than the boundary work. Finally, assume that the thermal energy stored in the cylinder itself is negligible and that the compression or expansion process is quasi-equilibrium. Consider the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary, closed system can be expressed as: E in − E out 14 24 3 Net energy transfer by heat, work, and mass = ∆E system 1 424 3 Change in internal, kinetic, potential, etc. energies Wb ,in − Qout = ∆U = m(u 2 − u1 ) (since KE = PE = 0) Qout = Wb ,in − m(u 2 − u1 ) From Tbl A-4: T m3 T1 = 200°C v 1 = v g = 0.12721 kg x1 = 1 u1 = u g = 2594.2 kJ kg P1 = P2 = 1554.9 kPa 2 1 3 v m T2 = 200°C v 2 = v f = 0.001157 kg kJ x2 = 0 u 2 = u f = 850.46 kg hw05soln.docx Name: HW #05 Due: 03/06/2012 Solution Set ES 346 4-34 5 of 12 The work done during this process is: 2 wb,out = ∫ P dV = P (v 2 − v 1 ) 1 = (1554.9 kPa)(0.001157 − 0.12721) m = − 196.0 kJ 3 1 kJ kg 1 kPa ⋅ m 3 kg That is: Ans wb,in = 196.0 kJ kg Substituting into the energy balance equation: q out = wb ,in − (u 2 − u1 ) = wb ,in + u fg = 196.0 kJ kg + 1743.7 kJ kg = 1940 kJ Ans kg hw05soln.docx Name: HW #05 Due: 03/06/2012 ES 346 Solution Set 4-44 6 of 12 Problem: Saturated R-134a vapor at 100°F is condensed at constant pressure to a saturated liquid in a closed piston–cylinder system. Calculate the heat transfer and work done during this process, in Btu/lbm. Solution: Use the following assumptions for this solution: 1. The cylinder is stationary and thus the kinetic and potential energy changes are zero. 2. There are no work interactions involved other than the boundary work. 3. The thermal energy stored in the cylinder itself is negligible. 4. The compression or expansion process is quasi-equilibrium. The system will consist of the contents of the cylinder. This is a closed system since no mass enters or leaves. The energy balance for this stationary, closed system can be expressed as: E in − E out 14 24 3 Net energy transfer by heat, work, and mass = ∆E system 1 424 3 Change in internal, kinetic, potential, etc. energies Wb ,in − Qout = ∆U = m(u 2 − u1 ) R-134 100°F (since KE = PE = 0) Q Qout = Wb ,in − m(u 2 − u1 ) From Tbl A-11E, the properties of the refrigerant at the initial and final states are: 3 T1 = 100°F v 1 = v g = 0.34045 ft lbm ⇒ x1 = 1 u1 = u g = 107.45 Btu lbm 3 v 2 = v f = 0.01386 ft lbm u 2 = u f = 44.768 Btu lbm T2 = 100°F ⇒ P1 = P2 = 138.93 psia x2 = 0 u fg = 62.683 Btu lbm h fg = 71.080 Btu lbm T 2 1 v hw05soln.docx Name: HW #05 Due: 03/06/2012 ES 346 Solution Set 4-44 7 of 12 The boundary work done during this process is: 2 wb,out = ∫ Pdv = P(v 2 − v 1 ) 1 3 = (138.93psia)(0.01386 − 0.34045)ft 1 Btu = −8.396 Btu lbm 5.404 psia ⋅ ft 3 lbm The negative sign indicates that this work is actually work done on the system. Therefore, for our energy balance considerations, we change it to read: wb,in = 8.396 Btu Ans lbm Substituting into energy balance equation gives: q out = wb ,in − (u 2 − u1 ) = wb ,in + u fg = 8.396 + 62.683 = 71.08 Btu Ans lbm Note that since the process is assumed to be quasi-equilibrium, the heat transfer may also be determined from: − q out = h2 − h1 q out = h fg = 71.080 Btu lbm since ∆U + Wb = ∆H. hw05soln.docx Name: HW #05 Due: 03/06/2012 Solution Set ES 346 4-66 8 of 12 Problem: Nitrogen gas to 20 psia and 100°F initially occupies a volume of 1 ft3 in a rigid container equipped with a stirring paddle wheel. After 5000 lbf·ft of paddle wheel work is done on the nitrogen, what is its final temperature? Solution: Assume that Nitrogen is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of 126.2 K (227.1 R) and 3.39 MPa (492 psia). Assume that the kinetic and potential energy changes are negligible. Finally, assume that constant specific heats at room temperature can be used for nitrogen. From Tbl A-1E & A-2Ea: cv = 0.177 Btu lbm ⋅ ° R 3 R = 0.3830 psia ⋅ ft Nitrogen 20 psia 100°F Wpw lbm ⋅ ° R Consider the nitrogen as the system. This is a closed system since no mass crosses the boundaries of the system. The energy balance for this system can be expressed as: E in − E out 14 24 3 = Net energy transfer by heat, work, and mass ∆E system 1 424 3 Change in internal, kinetic, potential, etc. energies Wpw,in = ∆U = mcv (T2 − T1 ) (since KE = PE = 0) Using the Ideal Gas Law, the mass of nitrogen is: P1V (20 psia)(1 ft 3 ) = = 0.09325 lbm RT1 (0.3830 psia ⋅ ft 3 )(560 °R) lbm ⋅ ° R Substituting into the energy balance: , Wpw,in = mcv (T2 − T1 ) m= (5000 lbf ⋅ ft) ( ) 1 Btu = (0.09325 lbm) 0.177 Btu (T − 560)°R lbm ⋅ °R 2 778.17 lbf ⋅ ft ⇒ T2 = 949° R = 489° F hw05soln.docx Ans HW #05 Due: 03/06/2012 Name: ES 346 Solution Set 4-126 9 of 12 Problem: A mass of 5 kg of saturated liquid–vapor mixture of water is contained in a piston–cylinder device at 125 kPa. Initially, 2 kg of the water is in the liquid phase and the rest is in the vapor phase. Heat is now transferred to the water, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 300 kPa. Heat transfer continues until the total volume increases by 20 percent. Determine (a) the initial and final temperatures, (b) the mass of liquid water when the piston first starts moving, and (c) the work done during this process. Also, show the process on a P-v diagram. Solution: Assume that the process is quasi-equilibrium. (a) Initially the system is a saturated mixture at 125 kPa pressure, and thus the initial temperature is found in Tbl A-5 to be: T1 = Tsat@125 kPa = 105.97°C Ans The total initial volume is: V1 = m f v f + m g v g 3 + (3kg )1.3750 m 3 = (2kg ) 0.001048 m kg kg = 4.127 m 3 P 2 3 1 v hw05soln.docx HW #05 Due: 03/06/2012 Name: ES 346 4-126 Solution Set 10 of 12 The total and specific volumes at the final state are: V 3 = 1.2V1 = (1.2 )(4.127m 3 ) = 4.953 m 3 V3 3 4.953 m 3 v3 = = = 0.9905 m kg 5 kg m Interpolating from Tbl A-6: , 3 T3 = 373.6°C m v3 = 0.9905 kg P3 = 300 kPa Ans (b) When the piston first starts moving, P2 = 300 kPa and V2 = V1 = 4.127 m3. The specific volume at this state is: v2 = V2 m = 3 4.127 m 3 = 0.8254 m kg 5 kg which is greater than vg = 0.60582 m3/kg at 300 kPa. Thus no liquid is left in the cylinder when the piston starts moving. Ans (c) No work is done during process 1-2 since V1 = V2. The pressure remains constant during process 2-3 and the work done during this process is: 3 Wb = ∫ P dV = P2 (V 3 − V 2 ) 2 1 kJ = 247.6kJ = (300 kPa )(4.953 − 4.127 )m 3 3 1 kPa ⋅ m Ans hw05soln.docx Name: HW #05 Due: 03/06/2012 ES 346 4-155 Solution Set 11 of 12 Problem: Two 10-ft3 adiabatic tanks are connected by a valve. Initially, one tank contains water at 450 psia with 10 percent quality, while the second contains water at 15 psia with 75 percent quality. The valve is now opened, allowing the water vapor from the high-pressure tank to move to the low-pressure tank until the pressure in the two becomes equal. Determine the final pressure and the final mass in each tank. Solution: Assume that the system is stationary and thus the kinetic and potential energy changes are zero and that there are no heat or work interactions involved. Consider both tanks to be the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary, closed system can be expressed as: E in − E out 14 24 3 = Net energy transfer by heat, work, and mass ∆E system 1 424 3 Change in internal, kinetic, potential, etc. energies 0 = ∆U = U 2 − U 1 = 0 U1 = U 2 m1, A u1, A + m1, B u1, B = m2, A u 2, A + m2, B u 2, B where the high-pressure and low-pressure tanks are denoted by A and B, respectively. From Tbl A-5E, the specific volume in each tank can be found: 3 3 3 P1, A = 450 psia v 1, A = v f + xv fg = 0.01955 ft + (0.10)(1.0324 − 0.01955) ft = 0.12084 ft lbm lbm lbm Btu Btu Btu x1, A = 0.10 + (0.10)(683.52) = 504.02 u1, A = u f + xu fg = 435.67 lbm lbm lbm hw05soln.docx HW #05 Due: 03/06/2012 Name: 4-155 Solution Set 12 of 12 ES 346 3 3 3 P1, B = 15 psia v 1, B = v f + xv fg = 0.01672 ft + (0.75)(26.297 − 0.01672) ft = 19.727 ft lbm lbm lbm Btu Btu x1, B = 0.75 u1, B = u f + xu fg = 181.16 Btu + (0.75)(896.52) = 853.55 lbm lbm lbm The total mass contained in both tanks is: VA VB 10 ft 3 10 ft 3 m= + = + = 83.26 lbm 3 v 1, A v 1, B 0.12084 ft 3 19.727 ft lbm lbm Similarly, the initial total internal energy contained in both tanks is: U 1 = m1, A u1, A + m1, B u1, B = (82.75lbm)(504.02 Btu lbm ) + (0.5069lbm)(853.55 Btu lbm ) = 42,155 Btu The specific internal energy and the specific volume are: u1 = u 2 = U 42,155 Btu = = 506.3 Btu lbm m 83.26 lbm 3 20 ft 3 = 0.2402 ft v1 = v 2 = = lbm m 83.26 lbm V Now, the final state is fixed. The pressure in this state may be obtained by iteration in Tbl A-5E after estimating the starting point from the Mollier Diagram: P2 = 313 psia Ans Since the specific volume is now the same in both tanks, and both tanks have the same volume, the mass is equally divided between the tanks at the end of this process, m 83.26 lbm m 2, A = m 2, B = = = 41.63lbm 2 2 hw05soln.docx Ans