Home Exam (I) for CHE 303 Course ,first semester 2015/2016 Q1
advertisement

Home Exam (I) for CHE 303 Course ,first semester 2015/2016
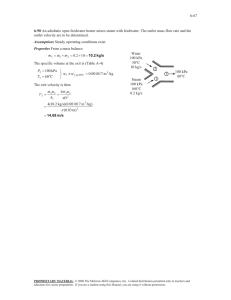
Q1Water at 75°C and V(specific volume )1.026 cm3/g-3 is heated up to (M+N+L)*25°C. The Pressure of the
heated water is 657 Kpa. Calculate the changes in : P,T,S,H,V for this process.
Q2Wet steam at 108 C and quality of 80% is cooled under constant Temperature. the change in the
enthalpy of the cooled steam is (M+N+L) *80 kj/kg. what is the final quality of this steam? What are the
changes in all other thermodynamic properties?.
Q3Determine the thermodynamic state of water at:
a- P=40 Kpa. And T=(M+N)*11 °C , b-T=525°C and P=725 Kpa.
c –P = (650-M-N-L) Kpa and T=425°C ?
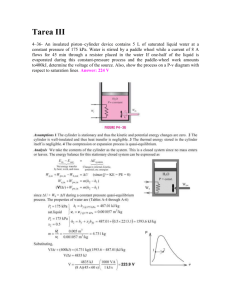
Q4-Methane (CH4) at -140°F and a specific volume of (M+N+L)/4 ft3/lbm is to be compressed
isothermally until the pressure is 425 Psia. and Enthalpy of 140 Btu/lbm. For this process ,determine the
expected changes in all thermodynamic properties. What is the value of θV/θP)T , θH/θV)T in the
Q5- Tetraflouroethane (HFC-134 a ) refrigerant undergoes a change in state from saturated liquid at
20°F to a final state at T=20°F and 4psia.determine the changes in its thermodynamic properties Specific
Volume ,P ,Enthalpy , Entropy ?
superheated vapor region .
Q6-Repeat example 4-8 page145 of the text book.with the following changes :
The products reach a temperature of Σ(I.D)* 35 K and that reactants are to be preheated to Σ(I.D)*35/2
K. and that the product stream contains {Σ(I.D)/2.1}% CO?
Good luck
Submission ,before 19/sep.2015
Dr. Jamal Amous