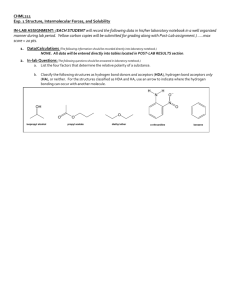
ch3nhch3 ch3coch3 ch3conhch2ch3

C341/ Chapter 1: General Chemistry Review sheet
1.
For the condensed formula below, perform the following functions: a.
Draw the correct Lewis structures and fill in the correct number of lone pairs and/or double bonds.
b.
Determine the molecular shape around each non ‐ hydrogen atom.
(tetrahedral, bent, etc.)
c.
Determine ideal bond angles and identify any distortions from ideal bond angles.
d.
Provide an orbital hybridization description for any atom and be able to describe the bonds in terms of overlapping atomic orbitals (sigma, pi, etc.).
In what types of orbitals do lone pairs reside?
f.
Which molecules are polar?
Which molecules would be soluble in polar solvents?
Draw in the δ + and δ‐ signs for the polar bonds.
g.
Finally, draw the correct lines structures for these compounds and identify the functional groups in each.
CH
3
NHCH
3
CH
3
COCH
3
CH
3
CONHCH
2
CH
3
Page 1 of 6
CH
2
CHCOCH
3
C341/ Chapter 1: General Chemistry Review sheet
(CH
3
)CHC(CH
3
)
2
(no polar bonds below)
CHCCH
2
CH
2
COOCH
3
(not all bonds are described because they are SO similar to previous molecules)
C(sp
2
) -- O(sp
3
) overlap sp
2
, trigonal planar, 120 o
C(sp) -- C(sp
3
) overlap sp
3
, bent, <109.5
o
H H O H H H O H
H H C C C C C O C sp, linear, <180 o
H H H sp
3
, tetrahedral, 109.5
o
H H C C C C C O C
H H
C(sp
3
) -- H(s) overlap
σ
: C(sp) -- C(sp) overlap
π( x
)
: C(2p) -- C(2p) overlap
π( y
)
: C(2p) -- C(2p) overlap
H
O
O
Page 2 of 6
C341/ Chapter 1: General Chemistry Review sheet
2.
Identifying conjugation .
The first step to being able to draw resonance structures is to identify conjugated atoms.
On the structure of capsaicin below circle all of the conjugated atoms.
Capsaicin is the active component to chili peppers.
The spicy flavor of capsaicin comes from its interaction with olfactory and other receptors.
The strength of the interaction is controlled by a variety of factors, including electrostatic interactions, which might arise from uneven electron distribution in the capsaicin molecule.
Resonance influences this electron distribution.
3.
Practicing resonance .
The protein content of a food sample is estimated by determining its nitrogen content.
The apparent protein content of some wheat gluten was boosted by the addition of melamine.
While melamine is rich in nitrogen, it causes kidney failure if the amount is high enough.
Polymerization of melamine with formaldehyde
(H
2
C=O) gives melamine resin, a tough plastic used in kitchen countertops and bowls.
Using curved arrows, provide two more resonance structures for melamine.
4.
Utilize arrows properly to show the formation of all other resonance structures if they exist.
Circle the
most stable resonance structure for each below.
a.
All resonance structures are completely equivalent.
Positive sign is on 2 o carbon in all instances and all have
the same number of bonds.
b.
Page 3 of 6
c.
d.
C341/ Chapter 1: General Chemistry Review sheet
No
resonance
structures
possible
because
the
charge
and
the
double
bond
are
not
in
conjugation
with
each
other.
e.
f.
5.
Determine the functional groups below.
Explain why the first two compounds which have the same molecular formula, C
4
H
10
O, have such different boiling points.
How would you expect 2 ‐ butanone boiling point to compare to these relatively?
Discuss their order of solubility in water.
Compound O
Boiling
Water
Point
Functional
Group
Intermolecular
Solubility
Forces present
108 o
C
1 o
alcohol
LF, DD, HB
Most soluble due to
HBA and HBD
34.6
ether
LF,
Least and o
DD
C
(but minor) soluble least DD
due
to HBA
80 ketone
LF, o
C
DD
HBA diethyl
and
(more
higher ether
significant)
DD
than
Boiling point : Doing this worksheet, you can always look up the boiling point for 2 ‐ butanone online with a quick Google search – that’s not the point of this question.
On an exam or quiz, you want to be able to deduce which compound has the most and least number of intermolecular forces.
The more intermolecular forces, or attractive forces between each other, it has then the higher its physical properties like boiling point.
By understanding this concept, you should understand that the ketone will have the intermediary boiling point because it has intermediary intermolecular forces.
Both the ether and the ketone have DD forces, BUT the ether has two polar bonds that help cancel each other out better due to the bond angles it experiences around the oxygen that is sp
3
and 109.5
bond angle.
Water solubility : If a molecule has more heteroatoms (F, N and O) it can hydrogen bond with water more effectively and will have higher water solubility.
All three molecules have one oxygen, so this concept will not be the deciding factor in the choice.
Only the alcohol has a HBD and HBA to bond with water.
The ketone and ether only have HBA and these two molecules will not hydrogen bond as effectively with water, hence, lower water solubility.
Page 4 of 6
C341/ Chapter 1: General Chemistry Review sheet
6.
Draw line carbons
as drawings
primary,
for all isomers secondary
and of
molecular tertiary.
If
formula you drew
C
4 an
H
10
O.
Identify the functional groups and discuss the
alcohol, is it a 1 o
, 2 o
, or 3 o
?
I may be missing some isomers , but you get the point.
7.
Write the Lewis structures and condensed structural formulas for all isomers of molecular formula C
4
H
8
O.
Identify the functional groups and discuss the carbons as primary, secondary and tertiary.
O
O
O ether ether 2 o alcohol
OH
3 o
OH alcohol O ketone O alkene + 1 o
OH alcohol alkene + 1 o
OH alcohol aldehyde
H
H aldehyde alkene + 1 o
OH alcohol
OH alkene + 2 o alcohol
OH alkene + 1 o alcohol alkene + 2 o
OH alcohol
O O
O ethers + alkene
8.
Fill in the lone pairs on all heteroatoms in each compound and assign correct formal charges.
O
Page 5 of 6
C341/ Chapter 1: General Chemistry Review sheet
9.
Identify as many functional groups as possible and then list the intermolecular forces (LF, DD, HBA, HBD) found in each of the following molecules.
What is the five carbon rule?
Predict whether the following molecules would be soluble in water.
Aspirin ( water soluble, LF, DD, HBA, HBD )
Vitamin C ( water soluble, LF, DD, HBA, HBD )
Melatonin ( water soluble, LF, DD, HBA, HBD alcohol, specifically called an aryl alcohol
HO arene alkane or hydrocarbon
O ether
Vitamin E (fat soluble, primarily LF)
)
Vitamin B
6
( water soluble, LF, DD, HBA, HBD )
alkane alkenes
HO secondary alcohol
Vitamin D (fat soluble, primarily LF, but does have minimal DD and HBA, HBD)
Dimitone ( water soluble, LF, DD, HBA, HBD )
N amine alkene
O carboxylic acid
OH
NH
2 primary amine corrected 1/20/10
Sabril – seizure medication
( water soluble, LF, DD, HBA, HBD )
Cl arene amide
aryl halide
Claritin – treats seasonal allergies
( fat soluble, LF, DD, HBA )
Page 6 of 6
N
O
O ester