Chapter 9: Addition Reactions of Alkenes
advertisement

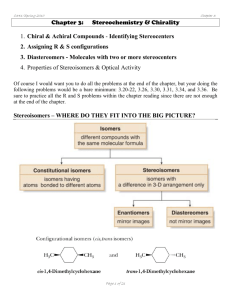
C341/Fall 2011 Chapter 9: Addition Reactions: Chapter 9: Addition Reactions of Alkenes 1. Addition vs. Elimination 2. Addition Reactions to Alkenes A. Addition of HX (hydrohalogenation) B. Addition of H2O (acid catalyzed hydration) C. Addition of H2O (oxymercuration‐demercuration) D. Hydroboration‐Oxidation E. Catalytic Hydrogenation F. Addition of X2 (halogenation) G. Addition of X2/H2O (halohydrin) H. Anti‐hydroxylation I. Syn‐hydroxylation J. Oxidative Cleavage 3. Synthesis Strategies (intro to one‐step and multi‐step syntheses) It would be BEST if you did ALL the textbook problems, but at the very least do these: 9.49‐9.55, 9.57, 9.58, 9.60, 9.63‐9.66, 9.68‐9.71, 9.74‐77, 9.79, 9.81, 9.82 Page 1 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Addition Reactions: Try to minimize any memorization and focus on understanding the mechanisms and learn to understand why reactions occur as they do. DO NOT JUST MEMORIZE THEM! Page 2 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: 1. Addition vs. Elimination – • Addition is the opposite of elimination (a pi bond is converted to a sigma bond). • Because an addition is the reverse of an elimination, often the processes are at equilibrium. • One must assess the ΔG to determine which side the equilibrium will favor. • A pi bond will often act as a Lewis base (i.e. nucleophile or as a Brønsted‐Lowry base). Page 3 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Thermodynamics of Addition Reactions • Typical addition reactions have a –ΔH. • What will the sign (+/‐) be for ΔSsys? • Predict the sign for ΔG. What is the driving force of addition reactions to alkenes? Page 4 of 36 C341/Fall 2011 2. Chapter 9: Addition Reactions: Addition Reactions Alkenes are nucleophiles, so we will react them with electrophilic reagents. In 1869, Markovnikov made an important observation for all addition reactions: Markovnikov addition = Regioselective reaction = Syn versus anti addition = Page 5 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: A. Hydrohalogenation – Electrophilic Addition of HX Markovnikov Addition of HBr Anti‐Markovnikov Addition of HBr H Br ROOR Page 6 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Predict the products for the following reactions: Page 7 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Provide the mechanism explaining the following reaction: Page 8 of 36 C341/Fall 2011 B. Chapter 9: Addition Reactions: Hydration — Acid‐Catalyzed Hydration of Water What do you observe about this reaction? Could you use these conditions to make primary alcohols? Practice drawing products before we try the mechanism: Page 9 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Acid‐catalyzed hydration mechanism: A catalyzed reaction mechanism always regenerates the catalyst. Page 10 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Acid‐catalyzed hydration (addition) and elimination are in equilibrium What might one do to shift the equilibrium toward the side you desire? Page 11 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: C. Oxymercuration‐demercuration Alternative that provides products more cleanly with no rearrangements. Reaction is: Reagents are: 1. Hg(OAc)2 is: 2. NaBH4 is: o This reaction is regiospecific o Markovnikov addition with NO rearrangement. Page 12 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Mechanism? How would the product be different if the reaction was done in ethanol instead of water? Page 13 of 36 C341/Fall 2011 D. Chapter 9: Addition Reactions: Hydroboration‐Oxidation (last type of hydration of an alkene) What do you notice about the addition above? What is BH3(THF)? BH3 does not exists as a single species, but it exists as a Lewis acid/Lewis base adduct: Page 14 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: 1) BH3 THF 2) H2 O2 / NaOH Page 15 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Hydroboration Mechanism: Page 16 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Compare all three hydration reaction conditions: Page 17 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: E. Catalytic Hydrogenation Most alkenes react with H2 in the presence of a late transition metal catalyst (Pd, Pt, Ru or N) at high pressure (ca. 3 atm or higher). Yields are nearly quantitative (i.e. ca. 99%). Heterogeneous Catalysis: Page 18 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Homogeneous Catalysis • In 1968, Knowles modified Wilkinson’s catalyst by using a chiral phosphine ligand. • A chiral catalyst can produce one desired enantiomer over another. • Why would someone want to synthesize one enantiomer rather than a racemic mixture? Page 19 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Noyori and Knowles shared the 2001 Nobel Prize in Chemistry for asymmetric catalysis: Page 20 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: F. Halogenation—Addition of X2 • Halogenation with Cl2 and Br2 is generally effective, but halogenation with I2 is too slow, and halogenation with F2 is too violent. • The reaction can be run “neat” or in an “inert” solvent like CCl4 or CH2Cl2. • Halogenation occurs with ANTI addition Predict the major product(s) for the reactions below. Br2 CCl4 Br2 H2O Page 21 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Mechanism? Page 22 of 36 C341/Fall 2011 G. Chapter 9: Addition Reactions: Halohydrin Reaction ‐ Addition of X2/H2O What observation(s) do you notice about how the substituents added? Mechanism? Page 23 of 36 C341/Fall 2011 H. Chapter 9: Addition Reactions: Anti‐dihydroxylation What are peroxy acids? (e.g. mCPBA) Replacing the relatively unstable O–O single bond is the thermodynamic driving force for this process. Is there anything unstable about an epoxide? Page 24 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Acid‐catalyzed ring opening mechanism: Page 25 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Note the similarity in all these intermediates: • Ring strain and a +1 formal charge makes these structures GREAT electrophiles. • They also each yield ANTI products because the nucleophile must attack in an SN2 fashion. Page 26 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: I. Syn‐dihydroxylation • Like other syn additions, SYN dihydroxylation adds across the C=C double bond in ONE step. • Because OsO4 is expensive and toxic, conditions have been developed where the OsO4 is regenerated after reacting, so only catalytic amounts are needed. Page 27 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: I. Syn‐dihydroxylation • MnO41‐ is similar to OsO4 but more reactive. • SYN dihydroxylation can be achieved with KMnO4 but only under mild conditions (cold temperatures). • Diols are often further oxidized by MnO41‐, and MnO41‐ is reactive toward many other functional groups as well. • The synthetic utility of MnO41‐ is limited. 1. OsO4 2. NaHSO3 Page 28 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: J. Oxidative Cleavage of Alkenes o C=C double bonds are also reactive toward oxidative cleavage. o Oxidative cleavage of an alkene breaks both the and bonds of the double bond to form two carbonyl compounds. o Cleavage with ozone (O3) is called ozonolysis. o Reductive workup: Zn (in H2O) or dimethylsulfide (CH3SCH3) o Oxidative workup: H2O2 in ROH Page 29 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Ozonolysis – cleavage of a C=C bond to form carbonyls 1. O3 2. (CH3)2S Page 30 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: 3. Synthesis Strategies A. Analyze the reagents used to determine what groups will be added across the C=C double bond. B. Determine the regioselectivity (Markovnikov or anti‐Markovnikov). C. Determine the stereospecificity (syn or anti addition) – Each step can be achieved with minor reagent memorization and a firm grasp of the mechanistic rational. – The more familiar you are with the mechanisms, the easier predicting products will be. What type of reaction type is depicted below? Page 31 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Practicing One‐Step Synthesis. Provide correct product(s) for the following reactions demonstrating correct stereochemistry in your products. If the product has an enantiomer or diastereomer, then just write +E or +D as necessary. Finally, circle if the reaction undergoes ANTI or SYN addition (if a mechanism undergoes both then circle both words). Provide reagents for reactions A and B. Page 32 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Using mechanistic arrows provide step‐by‐step mechanism. Page 33 of 36 C341/Fall 2011 Chapter 9: Addition Reactions: Multistep synthesis: Page 34 of 36 C341/Fall 2011 \ Chapter 9: Addition Reactions: Multistep synthesis: Page 35 of 36 C341/Fall 2011 \ Chapter 9: Addition Reactions: Multistep synthesis: Page 36 of 36