CHML212
advertisement

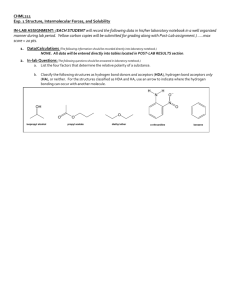
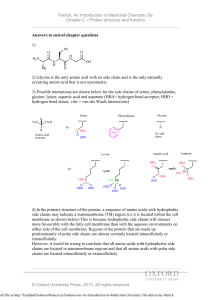
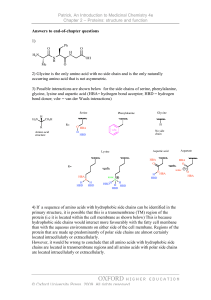
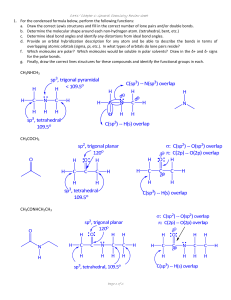
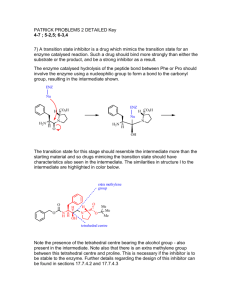
CHML212 Exp. 26 Synthesis and Analysis of Commercial Polymers IN-LAB ASSIGNMENT: (EACH STUDENT will record the following data in his/her laboratory notebook in a well organized manner during lab period. Yellow carbon copies will be submitted for grading along with Post-Lab assignment.) …..max score = 20 pts. 6. Data/Calculations a. b. c. d. Polyamide Synthesis i. Diameter of test tube ii. # test tube revolutions iii. Length of nylon produced calculation Linear Polyester Synthesis i. Mass of phthalic anhydride used ii. Mass of sodium acetate used iii. Volume of ethylene glycol used iv. Product appearance Cross-Linked Polyester Synthesis i. Mass of phthalic anhydride used ii. Mass of sodium acetate used iii. Volume of glycerol used iv. Product appearance Solubility Testing i. Total volume of toluene used ii. Total volume of acetone used iii. Total volume of methanol used 7. In-lab Questions (The following question should be answered in laboratory notebook.) a. b. Differentiate between a chain growth addition reaction and the step growth condensation reactions used to produce the polymers described in this experiment. Give an example of a polymer produced using each method. List the intermolecular forces present in polystyrene, toluene, and nylon. Explain, in terms of IMF, why polystyrene is soluble in toluene, but nylon 6,10 is not. CHML212 Exp. 26 Synthesis and Analysis of Commercial Polymers POST-LAB ASSIGNMENT: (EACH LAB GROUP will submit one copy of a typewritten, paragraph style report addressing all of the points listed below. Must be written using PAST TENSE, PASSIVE VOICE. ) …..max score = 50 pts. 8. Experimental (Write 1-2 paragraphs including all of the following. Do NOT present a bulleted outline.) What type of reaction did you perform? Describe your actual synthetic procedure for each polymer. Include names of any reactants used and desired product, as well as name of solvent and catalyst used (if any). Be sure to give actual volumes/masses of compounds used during the synthesis (not just what the lab manual tells you to use). Describe the analytical techniques used to evaluate the product, including actual any equipment, measurements, and calculations required. 9. Results (Complete tables electronically, where possible. Otherwise, complete by hand once copy/pasted into your document.) Table 26.1 Nylon Fiber Analysis Test tube diameter (mm) # of test tube revolutions Length of nylon (mm) Table 26.2 Physical Property and Solubility Results IMF Predicted Observed Solubility Polymer Type and Solvent (circle all Solubility 1 2 3 that apply) (circle all that Acetone Toluene Methanol apply) (Sol. or (Sol. or (Sol. or Insol.) Insol.) Insol.) Synthetic A Polyamide LDF DD 1 2 3 HBA HBD B Linear polyester LDF DD 1 2 3 HBA HBD C Cross-linked polyester LDF DD 1 2 3 HBA HBD D Polystyrene LDF DD 1 2 3 HBA HBD Natural E Starch LDF DD 1 2 3 HBA HBD F Cellulose LDF DD 1 2 3 HBA HBD Solvent 1 Acetone LDF DD ***In the final lab report, copy/paste this table into HBA HBD your document, and circle the appropriate IMF and 2 Toluene LDF DD solubility predictions by hand. Observed solubility HBA HBD entries can either be typewritten or handwritten.*** 3 Methanol LDF DD HBA HBD 10. Discussion (Write 1-2 pages addressing all of the following points.) How long was the string of nylon produced during your synthesis? How was the length calculated? Which co-reactant produced the more brittle polymer when treated with phthalic anhydride, ethylene glycol or glycerol? What is the major difference in structure between these co-reactants, and how does this affect the physical properties observed? Which polymers were soluble in toluene? Which in acetone? Which in methanol? Do any of the solubility results observed contradict predictions based on intermolecular forces present between solvents and solutes? Support your conclusion using actual intermolecular forces in your answer. Include a short comment addressing what could be done differently to improve the experimental results, if repeated.