n l ml ms - Patterson Science
advertisement
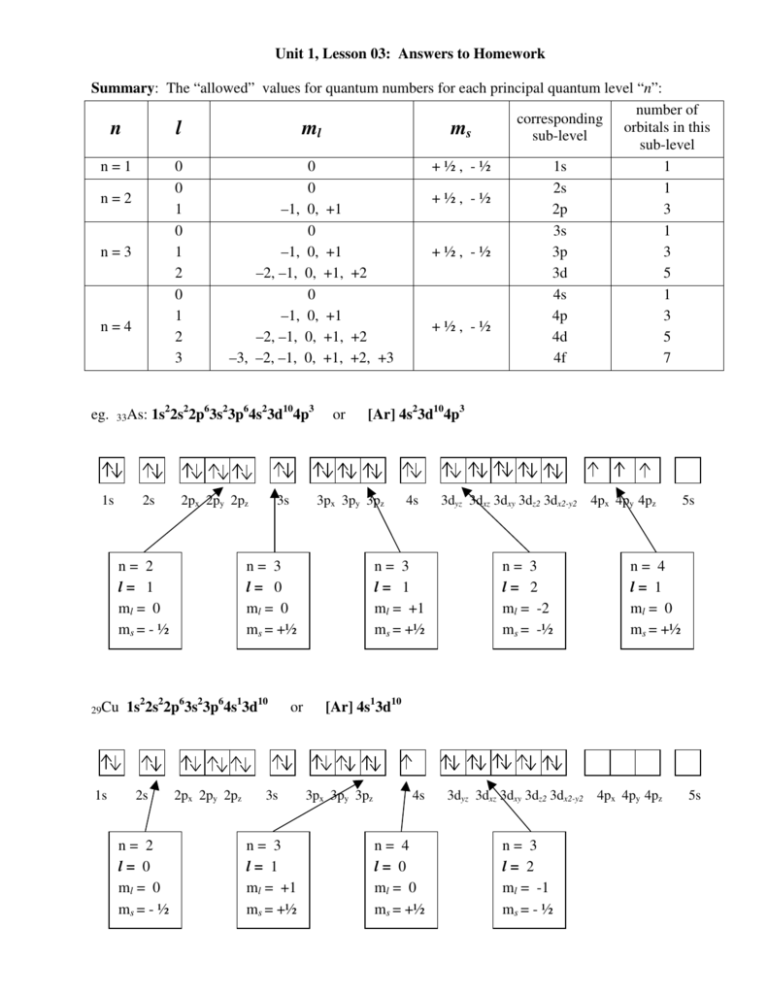
Unit 1, Lesson 03: Answers to Homework Summary: The “allowed” values for quantum numbers for each principal quantum level “n”: number of corresponding orbitals in this n l ml ms sub-level sub-level n=1 0 0 +½, -½ 1s 1 0 0 2s 1 n=2 +½, -½ 1 –1, 0, +1 2p 3 0 0 3s 1 n=3 1 –1, 0, +1 +½, -½ 3p 3 2 –2, –1, 0, +1, +2 3d 5 0 0 4s 1 1 –1, 0, +1 4p 3 n=4 +½, -½ 2 –2, –1, 0, +1, +2 4d 5 3 –3, –2, –1, 0, +1, +2, +3 4f 7 eg. 33As: 1s 1s22s22p63s23p64s23d104p3 2s 2px 2py 2pz n= 2 l= 1 ml = 0 ms = - ½ 29Cu 1s 3s n= 2 l= 0 ml = 0 ms = - ½ [Ar] 4s23d104p3 3px 3py 3pz n= 3 l= 0 ml = 0 ms = +½ 1s22s22p63s23p64s13d10 2s or 2px 2py 2pz or 3s n= 3 l= 1 ml = +1 ms = +½ 4s n= 3 l= 1 ml = +1 ms = +½ 3dyz 3dxz 3dxy 3dz2 3dx2-y2 4px 4py 4pz n= 3 l= 2 ml = -2 ms = -½ 5s n= 4 l= 1 ml = 0 ms = +½ [Ar] 4s13d10 3px 3py 3pz 4s n= 4 l= 0 ml = 0 ms = +½ 3dyz 3dxz 3dxy 3dz2 3dx2-y2 4px 4py 4pz n= 3 l= 2 ml = -1 ms = - ½ 5s Unit 1, Lesson 03: Homework on Quantum Numbers 1. Write the quantum numbers that represent the following electrons: a) a 5p3 electron would be given the quantum numbers: n = 5 , 2 l = 1 , ml = +1 and ms = + ½ b) a 3s electron would be given the quantum numbers: n = 3 , l = 0 , ml = 0 and ms = - ½ c) a 4f6 electron would be given the quantum numbers: n = 4 , l = 3 , ml = +2 and ms = + ½ 2. What are the allowable (possible) values for l when: a) n = 4: l can be 0, 1, 2 or 3 (s, p, d or f) b) n = 3: l can be 0, 1, or 2 (s, p or d) c) n = 1: l can be 0 (s) d) n = 5: l can be 0, 1, 2, 3 or 4 (s, p, d, f or g) 3. What are the allowable (possible) values for ml when: a) n = 4, l = 3: ml can be -3, -2, -1, 0, +1, +2, +3 b) n = 3, l = 1: ml can be -1, 0, +1 c) n = 2, l = 0: ml can be 0 d) n = 5, l = 4: ml can be -4, -3, -2, -1, 0, +1, +2, +3, +4 4. Write the principal quantum number and letter indicating orbital shape for each of the following: a) n = 2, l = 1 means 2p c) n = 4, l = 3 means 4f e) n = 4, l = 1 means 4p b) n = 3, l = 2 means 3d d) n = 1, l = 0 means 1s f) n = 2, l = 0 means 2s 5. State whether the following sets of quantum numbers are possible ( ) or impossible (X): a) n = 3, l = 3, ml= -1 and ms = + ½ no, when n = 3 then l has a maximum value of 2 (s, p or d) b) n = 5, l = 2, ml= -1 and ms = - ½ yes, this is the same as the 5d7 electron c) n = 2, l = 0, ml= 0 and ms = - ½ yes, this is the same as the 2s2 electron d) n = 3, l = 1, ml= 0 and ms = 0 no, ms must be either + ½ or – ½ e) n = 1, l = 0, ml= +1 and ms = + ½ no, when l = 0 (s) then the orientation ml must also be 0 f) n = 0, l = 0, ml= 0 and ms = + ½ no, n can not be 0 g) n = 4, l = 1, ml= +1 and ms = + ½ yes, this is the same as the 4p3 electron h) n = 2, l = 1, ml= -2 and ms = - ½ no, when l = 1 (p) then the orientation ml can be –1, 0 or +1 Unit 1, Lesson 03: Answers to Homework 1. Read pages 133 – 138. 2. On page 136, answer questions 1 – 5. Question 1: a) n = 5 What are the allowed values for l in each of the following cases? the allowed values for l are (0…n-1), so l can be 0, 1, 2, 3, 4 (double check: when n = 5, there are 5 types of orbitals: s, p, d, f, g) b) n = 1 the allowed values for l are (0…n-1), so l can be 0 (double check: when n = 1, there is 1 type of orbital: s) Question 2: What are the allowed values for ml for an electron with the following quantum numbers? a) l = 4 the allowed values for ml are (-l…0…+l), so ml can be –4, -3, -2, -1, 0, +1, +2, +3, +4 (double check: l = 4 means the same as the g sublevel, which can have 9 orbitals) b) l = 0 the allowed values for ml are (-l…0…+l), so ml can only be 0 (double check: l = 0 means the same as the s sublevel, which can have 1 orbital) Question 3: What are the names, ml values and total number of orbitals described by the following quantum numbers? a) n = 2, l = 0 this represents the second principal quantum level (n=2), and the “s” orbital: 2s -there is only one ml value: 0 because this represents only one orbital b) n = 4, l = 3 this represents the fourth principal quantum level (n = 4), and the ‘f’ orbitals: 4f -there are 7 ml values: -3, -2, -1, 0, +1, +2, +3 so this represents 7 orbitals Question 4: Determine the n, l and possible ml values for an electron in the 2p orbital -the 2 p orbital is in n = 2 -“p” orbitals are indicated by l = 1 -when l = 1, the allowed values for ml are –1, 0, +1 Question 5: Which of the following are allowable sets of quantum numbers for an orbital? Explain. a) n = 4, l = 4, ml = 0 this is not allowable because l can only have values up to (n - 1) b) n = 3, l = 2, ml = 1 this is allowable, l is less than n and ml can be any of –2, -1, 0, +1, +2 c) n = 2, l = 0, ml = 0 this is allowable, l is less than n and ml can only be 0 d) n = 5, l = 3, ml = -4 this is not allowable; ml can only have values –3, –2, -1, 0, +1, +2, +3 3. On page 138, answer questions 2, 3, 5, 6. Question 2: Quantum Number and Description 1. The Principal Quantum Number (n): • the allowed values for n are 1, 2, 3 …infinity 2. The Orbital Shape or Angular Momentum Quantum Number (l): • the allowed values for l are 0, 1, 2, 3 … (n – 1) 3. The Magnetic Quantum Number (ml): • the allowed values for ml are – l …. + l symbol n • l • ml • What it Describes describes the size of the quantum level or how far the electrons are from the nucleus (their energy) the shape of the energy sub-levels (types of orbitals) within each principal quantum level indicates the three dimensional orientation of an electron Question 3: n n=4 l 0 1 2 3 ml 0 –1, 0, +1 –2, –1, 0, +1, +2 –3, –2, –1, 0, +1, +2, +3 4s 4p 4d 4f 1 3 5 7 Question 5: Identify any values that are incorrect: a) n = 1, l = 1, ml = 0, name 1p When n = 1, the only allowable value for l is 0, which means ml is also 0 and indicates a 1s orbital b) n = 4, l = 3, ml = +1, name 4d The quantum numbers are correct, but the name is not. When l = 3, it indicates an “f” orbital, not d. c) c) n = 3, l = 1, ml = -2, name 3p The first two quantum numbers are correct and agree with the name. The value for ml is impossible: the allowed values when l = 1 are –1, 0, +1. Question 6: a) n = 4, l = 1, ml = 0, name 4p b) n = 2, l = 1, ml = 0, name 2p c) n = 3, l = 2, ml = -2, name 3d d) n = 2, l = 0, ml = 0, name 2s 4. Read pages 147 – 150. 5. To see how electron configurations are related to an element’s position on the periodic table, write the name of the last valence electron of each element (eg. 3d5) in the appropriate square of the Periodic Table below. Use the predicted electron configurations for Cr, Mo, W, Cu, Ag and Au. See next page 6. a) b) c) d) On the Periodic Table below, label the: Group numbers and Periods s,p,d and f blocks of elements the transition elements and inner-transition elements Noble gases, Alkali metals, Alkaline Earth metals, and Halogens Alkali metals Period ↓ Group→ 1 : 1 1s1 1s2 2 2s1 2s2 3 3s1 3s2 4 4s1 2 3 13 14 15 16 17 18 2p1 2p2 2p3 2p4 2p5 2p6 3p1 3p2 3p3 3p4 3p5 3p6 4s2 3d1 3d2 3d3 3d4 3d5 3d6 3d7 3d8 3d9 3d10 4p1 4p2 4p3 4p4 4p5 4p6 5 5s1 6 6s1 5s2 4d1 4d2 4d3 4d4 4d5 4d6 4d7 4d8 4d9 4d10 5p1 5p2 5p3 5p4 5p5 5p6 6s2 5d1 5d2 5d3 5d4 5d5 5d6 5d7 5d8 5d9 5d10 6p1 6p2 6p3 6p4 6p5 6p6 7s1 7s2 6d1 6d2 6d3 6d4 6d5 6d6 6d7 6d8 6d9 6d10 7p1 7p2 7p3 7p4 7p5 7p6 Inner Transition Elements (f block) 4 5 Noble ↓ gases Halogens ↓ Alkaline Earth metals 6 7 8 9 10 11 12 Transition Elements (d block) 4f1 4f2 4f3 4f4 4f5 4f6 4f7 4f8 4f9 4f10 4f11 4f12 4f13 4f14 5f1 5f2 5f3 5f4 5f5 5f6 5f7 5f8 5f9 5f10 5f11 5f12 5f13 5f14 7. On the page “Nuclear Charge and the Shielding Effect: Explaining the Trends on the Periodic Table” (handed out in class), for each element complete the: a) electron configuration b) Rutherford-Bohr diagram c) Nuclear charge (the number of protons in the nucleus = atomic number = Z) d) Shielding effect (the number of electrons in the full shells between the nucleus and the valence shell) e) Net Nuclear attraction (the nuclear charge subtract the shielding effect). Net nuclear attraction is the effective (Zeff) or actual attraction that exists between a valence electron and the nucleus. f) Use the numbers on the back of your Periodic Table to complete the ionization energy (First Ionization Potential, V), electronegativity and Atomic Radius (∆, Angstroms) Bring the completed sheet to class for our next lesson! Shielding Effect









