Ions, Formulas & Names: Chemistry Worksheet
advertisement
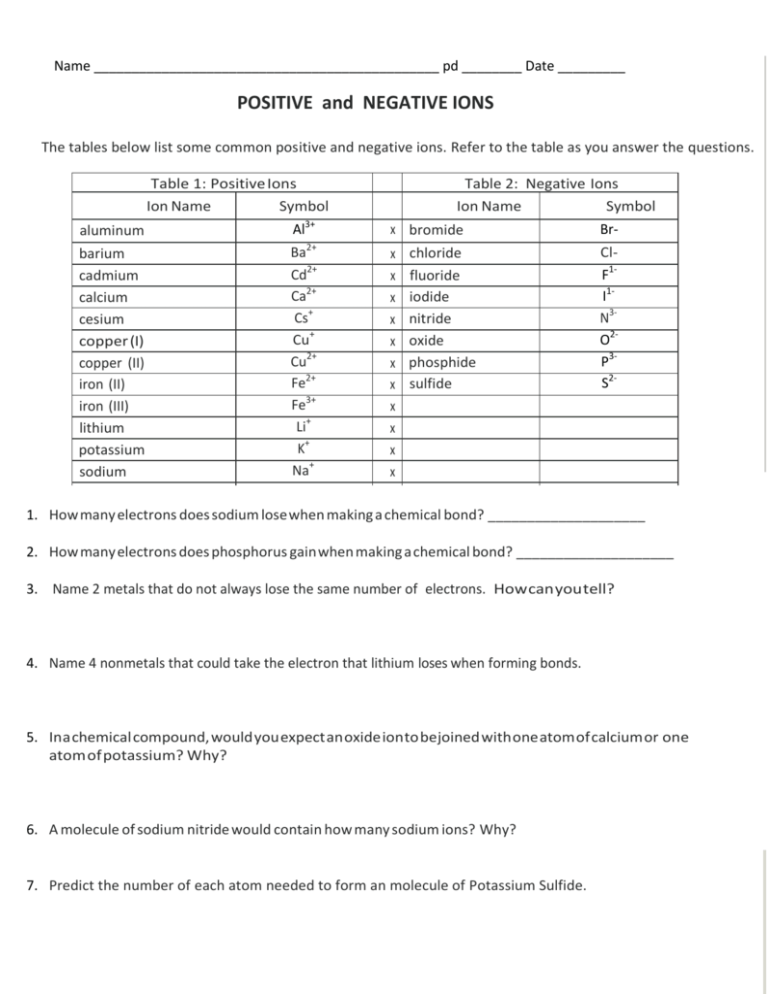
Name ______________________________________________ pd ________ Date _________ POSITIVE and NEGATIVE IONS The tables below list some common positive and negative ions. Refer to the table as you answer the questions. Table 1: Positive Ions Symbol Ion Name aluminum Al3+ barium cadmium calcium cesium copper (I) copper (II) iron (II) iron (III) lithium potassium sodium 2+ Ba Cd2+ Ca2+ Cs+ Cu+ Cu2+ Fe2+ Fe3+ Li+ K+ Na+ Table 2: Negative Ions Symbol Ion Name x bromide x x x x x x x x x x x chloride fluoride iodide nitride oxide phosphide sulfide BrClF1I1N3O2P3S2- 1. How many electrons does sodium lose when making a chemical bond? ____________________ 2. How many electrons does phosphorus gain when making a chemical bond? ____________________ 3. Name 2 metals that do not always lose the same number of electrons. How can you tell? 4. Name 4 nonmetals that could take the electron that lithium loses when forming bonds. 5. In a chemical compound, would you expect an oxide ion to be joined with one atom of calcium or one atom of potassium? Why? 6. A molecule of sodium nitride would contain how many sodium ions? Why? 7. Predict the number of each atom needed to form an molecule of Potassium Sulfide. Name ______________________________________________ pd ________ Date _________ NAMES AND FORMULAS WORKSHEET #1 Write the correct formula for each of the following compounds. 1. lithium iodide 2. calcium oxide 3. sodium sulfide 4. aluminum oxide 5. barium sulfide 6. aluminum chloride 7. lithium fluoride 8. cesium nitride 9. beryllium fluoride 10. potassium phosphide Give the correct name for each of the following ionic compounds. 11. LiI __________________________________________________ 12. Na2S 13. MgBr2 14. AIF3 15. BaO 16. BaI2 17. KCI 18. CaO 19. LiCI 20. BeS Name ______________________________________________ pd ________ Date _________ Formula Worksheet 2 1. strontium iodide _____________________ 2. rubidium nitride _____________________ 3. manganese (IV) chloride_____________________ 4. zinc fluoride_____________________ 5. chromium (Ill) oxide_____________________ 6. iron (II) phosphide_____________________ 7. cobalt (II) sulfide _____________________ 8. silver oxide _____________________ 9. mercury (I) chloride _____________________ 10. Mn2O3 _____________________ 11. ScO2 ____________________ 12. K2O ____________________ 13. NaCl _____________________ 14. ZnS_____________________ 15. Al2S3 ____________________ 16. Cr205 17. FeP _____________________ _____________________ Name ______________________________________________ pd ________ Date _________ NAMES AND FORMULAS WORKSHEET #3 Write the correct formula for each of the following c ompounds. 1. mercury (II) sulfide 2. zinc oxide 3. sodium sulfide 4. copper (I) oxide 5. iron (111) bromide 6. tin (IV) oxide 7. aluminum oxide 8. manganese (I) sulfide 9. cobalt (II) phosphide 10. silver iodide _ Give the correct name for each of the following ionic compounds. 11. Cr203 12. MnBr2 13. PbO 14. FeS 15. FeF2 16. Hgl 17. CuO 18. AgCl











