Worksheet 4 Notes - Oregon State University
advertisement
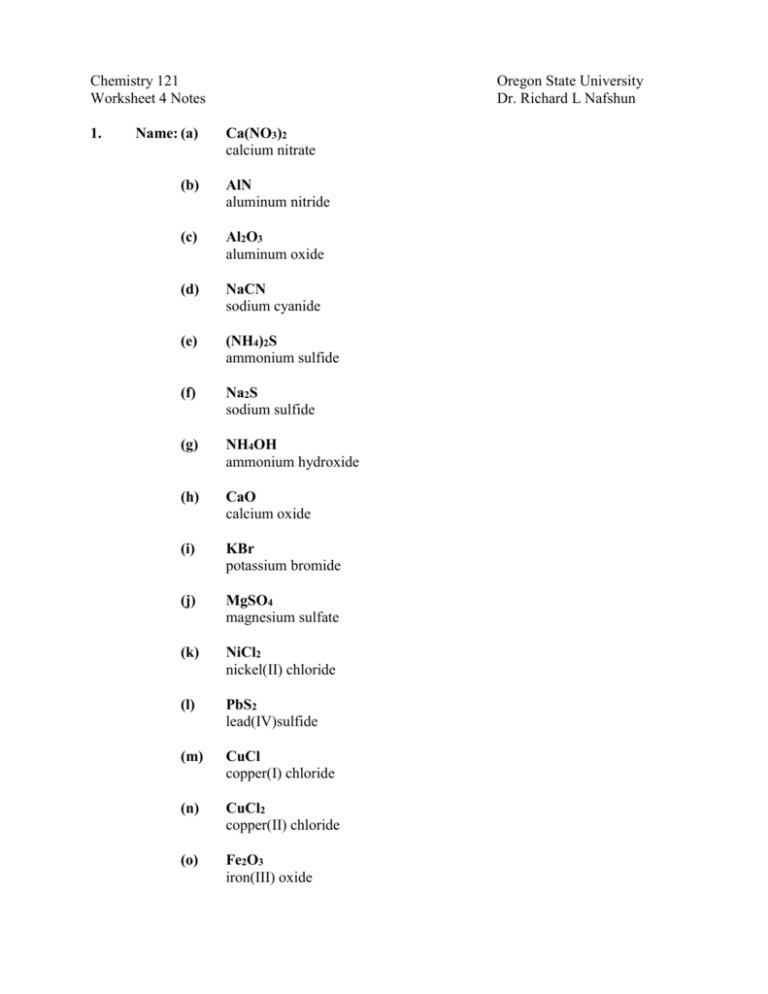
Chemistry 121 Worksheet 4 Notes 1. Name: (a) Oregon State University Dr. Richard L Nafshun Ca(NO3)2 calcium nitrate (b) AlN aluminum nitride (c) Al2O3 aluminum oxide (d) NaCN sodium cyanide (e) (NH4)2S ammonium sulfide (f) Na2S sodium sulfide (g) NH4OH ammonium hydroxide (h) CaO calcium oxide (i) KBr potassium bromide (j) MgSO4 magnesium sulfate (k) NiCl2 nickel(II) chloride (l) PbS2 lead(IV)sulfide (m) CuCl copper(I) chloride (n) CuCl2 copper(II) chloride (o) Fe2O3 iron(III) oxide (p) 2. 3. FeO iron(II) oxide Give the chemical formulae for the following ionic compounds: (a) aluminum sulfide Al2S3 (b) sodium sulfide Na2S (c) sodium sulfate Na2SO4 (d) sodium hydroxide NaOH (e) aluminum cyanide Al(CN)3 (f) calcium nitrate Ca(NO3)2 (g) cesium phosphate Cs3PO4 (h) cerium(III) chloride CeCl3 (i) lithium acetate LiCH3COO (j) copper(II) carbonate CuCO3 (k) iron(II) phosphate Fe3(PO4)2 (l) iron(III) oxide Fe2O3 Consider a red light that has a wavelength of 710.2 nm. Express this wavelength in cm. Express this wavelength in km. Express this wavelength in inches. 1m 100cm 710.2 nm = 7.102 x 10-5 cm 9 1x10 nm 1m 1m 7.102 x 10-5 cm 100cm 1km = 7.102 x 10-10 km 1000 m 1inch 7.102 x 10-5 cm = 2.796 x 10-5 inch 2.54cm 4. A student measures a mass to be 2.55 x 103 kg. Express this mass in mg. 1000 g 1000mg = 2.55 x 109 mg 2.55 x 103 kg 1kg 1g 5. What is a heterogeneous mixture? A mixture that is different throughout. What is a homogeneous mixture? A mixture that is the same throughout. How many elements are there? About 112 to 114. Approximately how many compounds have cataloged and characterized? About 17 million. Is heptane one of them? Yes. C7H16. Is aluminum chloride one of them? Yes. AlCl3. 6. Determine the number of protons, neutrons, and electrons in each of the following: Species 187 Os Au 198 Au3+ 198 Au+ 210 Pb 210 Pb2+ 7 Li 7 + Li 16 O 16 2O 18 O 18 2O 19 F 19 F 238 U 198 7. 76 79 79 79 82 82 3 3 8 8 8 8 9 9 92 e- n 111 119 119 119 128 128 4 4 8 8 10 10 10 10 146 76 79 76 78 82 80 3 2 8 10 8 10 9 10 92 List three isotopes of hydrogen. Identify the number of protons, neutrons, and electrons in each. Identify the number of protons, neutrons, and electrons in 1H+. Species 1 H 2 H 3 H 1 + H 8. p p 1 1 1 1 n 0 1 2 0 e1 1 1 0 Write the formula and name a compound composed of calcium and fluorine. CaF2 is calcium fluoride 9. Write the formula and name a compound composed of aluminum, sulfur, and oxygen. Al2(SO4)3 is aluminum sulfate 10. Natural strontium consists of the following isotopes: Isotope Strontium-84 Strontium-86 Strontium-87 Strontium-88 Molar Mass, u 83.913 85.909 86.909 87.906 Percent Abundance 0.56 9.86 7.00 82.58 Calculate the molar mass of strontium. The molar mass is the weighted average: Molar mass = (83.913 g/mol)(0.0056) + (85.909 g/mol)(0.0986) + (86.909 g/mol)(0.070) + (87.906 g/mol)(0.8258) = 87.634 g/mol











