Chapter 7 - Atomic radii
advertisement
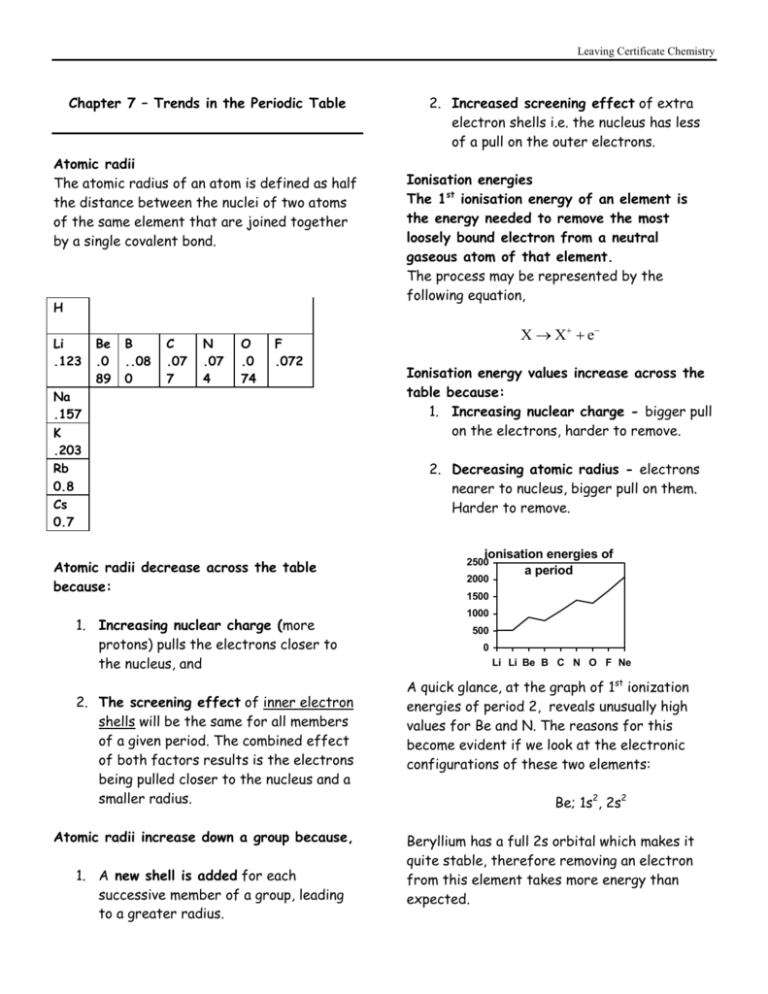
Leaving Certificate Chemistry Chapter 7 – Trends in the Periodic Table Atomic radii The atomic radius of an atom is defined as half the distance between the nuclei of two atoms of the same element that are joined together by a single covalent bond. H Li .123 Be .0 89 B ..08 0 C .07 7 N .07 4 O .0 74 F .072 Na .157 K .203 Rb 0.8 Cs 0.7 Atomic radii decrease across the table because: 1. Increasing nuclear charge (more protons) pulls the electrons closer to the nucleus, and 2. The screening effect of inner electron shells will be the same for all members of a given period. The combined effect of both factors results is the electrons being pulled closer to the nucleus and a smaller radius. Atomic radii increase down a group because, 1. A new shell is added for each successive member of a group, leading to a greater radius. 2. Increased screening effect of extra electron shells i.e. the nucleus has less of a pull on the outer electrons. Ionisation energies The 1st ionisation energy of an element is the energy needed to remove the most loosely bound electron from a neutral gaseous atom of that element. The process may be represented by the following equation, X X e Ionisation energy values increase across the table because: 1. Increasing nuclear charge - bigger pull on the electrons, harder to remove. 2. Decreasing atomic radius - electrons nearer to nucleus, bigger pull on them. Harder to remove. ionisation energies of a period 2500 2000 1500 1000 500 0 Li Li Be B C N O F Ne A quick glance, at the graph of 1st ionization energies of period 2, reveals unusually high values for Be and N. The reasons for this become evident if we look at the electronic configurations of these two elements: Be; 1s2, 2s2 Beryllium has a full 2s orbital which makes it quite stable, therefore removing an electron from this element takes more energy than expected. Leaving Certificate Chemistry N; 1s2,2s2,2px1,2py1,2pz1 Nitrogen has three half-filled 2p orbitals, this makes it very stable and removal of an electron is more difficult than expected. Ionisation energy values decrease down a group because: 1. Increasing atomic radius - electrons further from nucleus, nuclear pull is less, easier to remove. 2. An extra shell is added for each successive member of a group, therefore the screening effect is greater as you go down a group. The outer electrons are further removed and more easily removed. Subsequent ionisations If you study the 1st and subsequent ionisation energy values of an element such as potassium, you will observe some significant jumps in the energy values as you remove more and more electrons. You will see a general increase as you remove each subsequent electron. 120000 100000 ionisation energy 80000 60000 40000 20000 11 th 13 th 15 th 17 th 19 th 9t h 7t h 5t h 3r d 1s t 0 electrons removed But, more significantly, huge jumps occur when removing the 2nd , 10th , and 18th electrons, as to do so means breaking into a completely full energy level. This is further evidence for the existence of energy levels. Trends in electronegativity values ‘The electronegativity of an element is a measure of the power of attraction, of an atom of that element, for the shared pair of electrons in a covalent bond.’ Electronegativity values increase across the table because: 1. The nuclear charge is increasing and 2. The atomic radius is decreasing. These two factors combined make it more difficult to remove an electron. Electronegativity values decrease down a group because: 1. Increasing atomic radius 2. Increased screening effect.











