Isomerism - Wa Ying College
advertisement

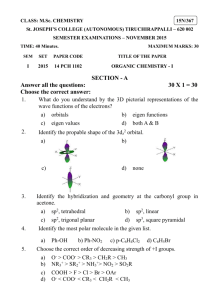
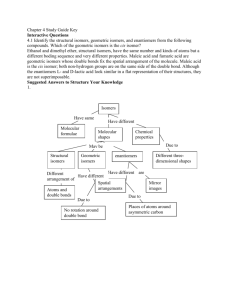
P.1 Isomerism A. Introduction The molecular formula (C4H10) has two display structural formulae: These two molecules are isomers, that is they have the same molecular formula but their atoms are arranged in a different way. For more example, propanoic acid and ethyl methanoate have the same molecular formula, C3H6O2. However, their structures and properties are different: There are many examples of isomerism in chemistry and these may be divided into two main classes: Structural isomerism Isomers whose molecules have their atoms linked together in different bonding arrangements B. Steroisomerism Isomers whose molecules have their atoms linked together in the same bonding arrangement but are arranged differently in space. Structural isomerism There are three main types : chain isomerism, positional isomerism and functional group isomerism. i. Chain isomers have different length of main hydrocarbon chain: For example C5H12 has two structural isomers 2-methylbutane 2,2-dimethylpropane Chain isomers have similar chemical properties but different physical properties as shown below as an example. properties pentane melting pt. boiling pt. density (g/cm3) -130 36 0.63 2-methylbutane -160 28 0.62 2,2-dimethylpropane -16 10 0.59 P.2 ii. Positional isomers have a particular atom, or bond, or group of atoms, in different molecular positions. For example, there are two positional isomers of molecular formula C3H7Br. Positional isomers have different physical properties. They take part in similar chemical reactions, but often at different rates. iii. Functional group isomers contain different organic functional groups. For example, propanoic acid and ethyl methanoate have the same molecular formula, C3H6O2. However, they have different functional groups, (acid and ester respectively). C. Stereoisomerism There are two types of stereoisomerism, geometrical isomerism and optical isomerism (enantiomerism). Stereoisomers are molecules which have different properties even though the same atoms are bonded together in the same way. Consider the molecular models of 1,2-dichloroethene. Each molecule has the same atoms linked together in the same way. However, their 3-D structures differ because the π electron cloud prevents rotation about the double bond. They are called geometrical isomers of but-2-ene. Consider the molecules (I) and (II) where W, X, Y and Z are all different. X X W W Z Z I II They are called enantiomers (mirror images of each other). Such stereoisomerism is called enantiomerism. P.3 D. Nomenclature of enantiomers: The name of the enantiomer is (R)-butan-2-ol. The carbon attaching with four different groups is called “chiral” carbon (or chiral centre). E. Optical activity and enantiomerism The following are some physical properties of (R)- and (S)-2-butanol Physical property Boiling point (oC at 1 atm) Density (g/cm3 at 20oC) Index of refraction (at 20oC) Specific rotation ([α] 25D ) (R) )-2-butanol 99.5 0.808 1.397 -13.52o Plane polarized light and optical activity: (S)-2-butanol 99.5 0.808 1.397 +13.52o P.4 Class work 1. Draw structural formulae for a. the four structural isomers of formula C3H6Cl2; b. the five structural isomers of formula C4H8; c. the four structural isomers of C4H9Br 2. Several compounds have the molecular formula C2H4O2. a. Three of these are functional group isomers containing a carbonyl group, C=O. Give their structures. b. Three other isomers contain a C=C bond. Draw their structural formulae. 3. Give the structural formulae for the isomers having the molecular formulae b. C5H10O (contains C=O) a. C3H6O2 (esters) 4.