Name: Date: Period: ______ IONIC BONDING BASICS PART A
advertisement

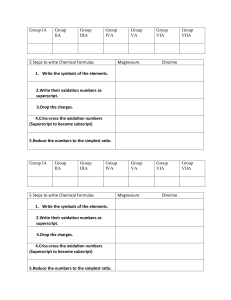
Name: __________________________ Date: _______________ Period: ___________ IONIC BONDING BASICS PART A: Show the transfer of electrons needed to form the ionic bond for each given elements. 1. Magnesium + Iodine 3. Aluminum + Chlorine 2. Beryllium + Chlorine 4. Potassium + Nitride NOTES: NAMING IONIC COMPOUNS 1. Name the cation (+) first 2. Name the anion (-) second and change the ending to –ide EXAMPLE: CAF2 the cation is Calcium, the anion is Fluorine, but you need to change the ending to –ide therefore it changes to fluoride YOUR ANSWER IS CALCIUM FLUORIDE FORMULA WRITING 1. Cross the oxidation numbers to get the final formula (TO GET SUBSCRIPTS) 2. Do not cross the (+) sign or the (-) Signs, only cross the numbers EXAMPLE: Ca+2 F-1 Ca1F2 YOU SHOULD NEVER SHOW A 1 IN THE FINAL FORMULA, THEREFORE YOUR FINAL ANSWER IS CaF2 PART B: Using the notes above, write the Formula and the Name for the ions given in Part A. Show your work to get the final formula (crossing of charges) 5. Magnesium + Iodine Formula: ________________________________ Name: ________________________________ 6. Beryllium + Chlorine Formula: ________________________________ Name: ________________________________ 7. Aluminum + Chlorine Formula: ________________________________ Name: ________________________________ 8. Potassium + Nitride Formula: ________________________________ Name: ________________________________ NOTES: Polyatomic ion: are groups of two or more elements that have an oxidation # associated with them as a group Examples: NH4+1 NO-3 Roman Numeral: Roman numerals indicate the ion’s charge Used when the ion has more than one oxidation state (Transition elements) Examples: Copper (II) Cu+2 Cobalt (III) Co+3 Part C: From the following list, circle the polyatomic ions C+4 PO4-3 OH- K+ HF- Part D: Given the following ions with Roman numerals, write the symbol with the oxidation number 9. Manganese (II) _______________________________ 10. Manganese (III) ______________________________ 11. Nickel (IV) _________________________________ 12. Gold (I) ____________________________________