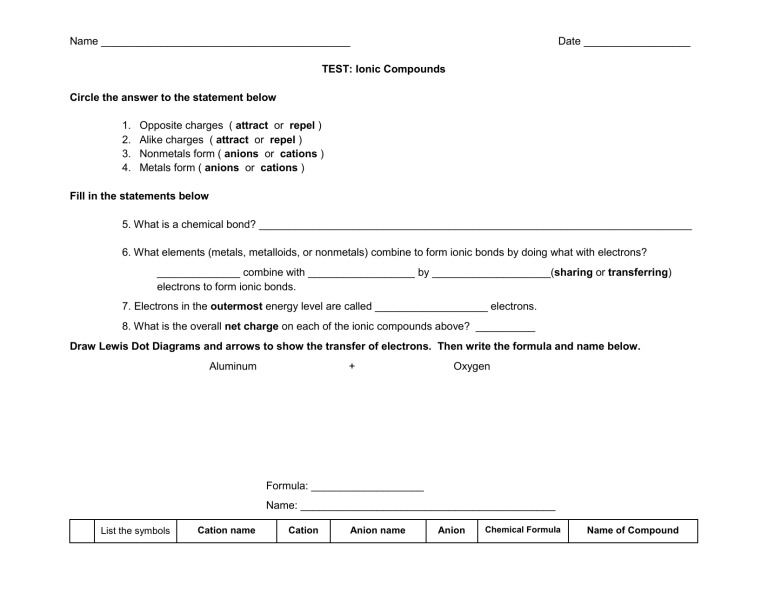
Name __________________________________________ Date __________________ TEST: Ionic Compounds Circle the answer to the statement below 1. 2. 3. 4. Opposite charges ( attract or repel ) Alike charges ( attract or repel ) Nonmetals form ( anions or cations ) Metals form ( anions or cations ) Fill in the statements below 5. What is a chemical bond? _________________________________________________________________________ 6. What elements (metals, metalloids, or nonmetals) combine to form ionic bonds by doing what with electrons? ______________ combine with __________________ by ____________________(sharing or transferring) electrons to form ionic bonds. 7. Electrons in the outermost energy level are called ___________________ electrons. 8. What is the overall net charge on each of the ionic compounds above? __________ Draw Lewis Dot Diagrams and arrows to show the transfer of electrons. Then write the formula and name below. Aluminum + Oxygen Formula: ___________________ Name: ___________________________________________ List the symbols Cation name Cation Anion name Anion Chemical Formula Name of Compound of the two ions and circle the metal 10 Na Cl 11 K O 12 symbol & oxidation number calcium Na1+ chloride symbol & oxidation number (Metal first) (Transition metals have oxidation number in parenthesis) Cl1- NaCl sodium chloride K2O Ca3P2 13 magnesium oxide 14 calcium bromide 15 aluminum sulfide 16 Fe2O3 17 Cu3N 18 CuO 19 iron (I) oxide 20 titanium (IV) chloride 21 chromium (III) nitride 22 Al2(SO4)3 23 NaNO3 24 (NH4)3PO4 25 sodium hydroxide 26 beryllium phosphate 27 ammonium sulfate





