Determination of Ka of Weak Acids
advertisement

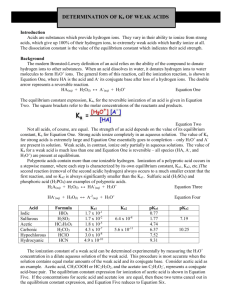
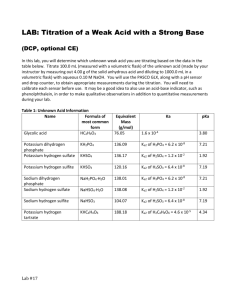
Determination of Ka of Weak Acids After Vonderbrink, Laboratory Experiments for AP Chemistry Introduction Acids are substances which provide hydrogen ions. They vary in their ability to ionize from strong acids, which give up 100% of their hydrogen ions, to extremely weak acids which hardly ionize at all. The dissociation constant is the value of the equilibrium constant which indicates their acid strength. Background The modern Bronsted definition of an acid relies on the ability of the compound to donate hydrogen ions to other substances. When an acid dissolves in water, it donates hydrogen ions to water molecules to form H+ ions. The general form of this reaction, called an ionization reaction, is shown in Equation 1, where HA is the acid and A− its conjugate base after loss of a hydrogen ion. The double arrows represent a reversible reaction. HA(aq) + H2O(l) A−(aq) + H3O+(aq) Ka = Equation 1 [A− ][H 3O + ] [HA] Equation 2 Not all acids, of course, are created equal. The strength of an acid depends on the value of its equilibrium constant Ka for Equation 1. Strong acids ionize completely in aqueous solution. The value of Ka for a strong acid is extremely large and Equation 1 essentially goes to completion -- only H3O+ and A− are present in € solution. Weak acids, in contrast, ionize only partially in aqueous solution. The value of Ka for a weak acid is much less than one and Equation 1 is reversible -- all species (HA, A—, and H3O+) are present at equilibrium. Polyprotic acids contain more than one ionizable hydrogen. Ionization of a polyprotic acid occurs in a stepwise manner, where each step is characterized by its own equilibrium constant (Ka1, Ka2, etc.). The second reaction (removal of the second acidic hydrogen) always occurs to a much smaller extent than the first reaction, and so Ka2 is always significantly smaller than Kal. Sulfuric acid (H2SO4) and phosphoric acid (H3PO4) are examples of polyprotic acids. H2A(aq) + H2O(l) HA−(aq) + H3O+(aq) K a1 = [HA− ][H 3O + ] [H 2 A] Equation 3 HA−(aq) + H2O(l) A2−(aq) + H3O+(aq) K a2 = [A2– ][H 3O + ] [HA– ] Equation 4 € The ionization constant of a weak acid can be determined experimentally by measuring the H3O+ concentration € in a dilute aqueous solution of the weak acid. This procedure is most accurate when the solution contains equal molar amounts of the weak acid and its conjugate base. Consider acetic acid as an example. Acetic acid (CH3COOH) and the acetate anion (CH3COO−) represent a conjugate acid-base pair. The equilibrium constant expression for ionization of acetic acid is shown in Equation 5. If the concentrations of acetic acid and acetate ion are equal, then these two terms cancel out in the equilibrium constant expression, and Equation 5 reduces to Equation 6. CH3COOH(aq) + H2O(l) CH3COO−(aq) + H3O+(aq) Ka = [CH 3COO – ][H 3O + ] [CH 3COOH] Equation 5 K a = [H 3O + ] Equation 6 In this experiment, solutions are prepared in which the molar concentrations of an unknown acid and its € conjugate base are equal. The pH of these solutions are then equal to the pKa for the acid. The definition of pKa is closely related to that of pH. Thus, pH = −log[H3O+] and pKa = −logKa. Most of the unknowns are salts of € polyprotic acids that still contain an ionizable hydrogen. Sodium bisulfate (NaHSO4 ), for example, is a weak acid salt: it contains Na+ and HSO4− ions. The HSO4− ion is a weak acid--the equilibrium constant for ionization of HSO4− corresponds to Ka2 for sulfuric acid. LabKaWeakAcid.docx 1 H2SO4(aq) + H2O(l) HSO4−(aq) + H2O(l) HSO4−(aq) + H3O+(aq) SO42−(aq) + H3O+(aq) K a1 = [HSO 4 - ][H 3O + ] [H 2SO 4 ] Equation 7 Ka2 = [SO 4 2− ][H 3O + ] [HSO 4 − ] Equation 8 € Experiment Overview The purpose of this experiment is to determine the pKa values for ionization of an unknown weak acid. A € solution containing an equal molar amount of the weak acid and its conjugate bases is prepared by "half neutralization" of the acid. The pH value is measured and used to calculate the pKa value for the unknown and thus determine its identities. Two trials are run. Procedure 1. Label two weighing dishes #1 and #2. 2. Obtain an unknown weak acid and record the code (letter) of the unknown. Measure out a small quantity (0.15-0.20 g) of the unknown into each weighing dish. Note: It is not necessary to know the exact mass of each sample. 3. Using a graduated cylinder, precisely measure 50.0 mL of distilled water into a 150-mL beaker. 4. Transfer the sample from weighing dish #1 (Trial 1) to the water in the beaker and stir to dissolve. 5. Using a graduated cylinder, precisely transfer 25.0 mL of the acid solution prepared in step 3 into an Erlenmeyer flask. Add 3 drops of phenolphthalein solution to the acid solution in the Erlenmeyer flask. 6. Using a Beral pipet, add sodium hydroxide solution dropwise to the flask. Gently swirl the flask while adding the sodium hydroxide solution. 7. Continue adding sodium hydroxide dropwise and swirling the solution until a faint pink color persists throughout the solution for at least 15 seconds. This is called the endpoint. Note: A pink color develops immediately when the base is added, but fades quickly once the solution is swirled. When nearing the endpoint, the pink color begins to fade more slowly. Proceed cautiously when nearing the endpoint, so as not to "overshoot" it. Note: At this point the solution in the beaker contains exactly one-half of the original amount of acid, essentially all of which is in the acid form, HA. The Erlenmeyer flask contains an equal amount of the conjugate base A− obtained by neutralization. 8. Pour the contents of the Erlenmeyer flask back into the beaker. Pour the solution back and forth a few times to mix. Note: It is important to transfer all of the solution from the Erlenmeyer flask back into the beaker. 9. Using a pH meter, measure the pH of the solution in the beaker, which now contains equal molar amounts of the acid and its conjugate base. Record the pH. 10. Dispose of the beaker contents down the drain and rinse both the beaker and the Erlenmeyer flask with distilled water. Dry the beaker with a paper towel. 11. Repeat steps 3-9 using the sample from weighing dish #2 (Trial 2). Pre-Lab Questions Phosphoric acid is a triprotic acid (three ionizable hydrogens). The values of its stepwise ionization constants are Ka1 = 7.5 × 10−3, Ka2 = 6.2 × 10−8, and Ka3 = 4.2 × 10−13. 1. Write the chemical equation for the first ionization reaction of phosphoric acid with water. 2. Write the equilibrium constant expression (Ka1) for this reaction. LabKaWeakAcid.docx 2 3. What would be the pH of a solution when [H3PO4] = [H2PO4−]? 4. What would be the pH of a solution prepared by combining equal quantities of NaH2PO4 and Na2HPO4? Explain with an equation. 5. Sufficient strong acid is added to a solution containing Na2HPO4 to neutralize one-half of it. What will be the pH of this solution? Explain. 6. Why is it not necessary to know the exact mass of your acid sample (step 2 in the Procedure) nor the exact concentration of the sodium hydroxide solution used (step 6 in the Procedure)? Why is it necessary to measure the exact volume of distilled water used to dissolve the acid (step 3) as well as the exact volume of the solution transferred from the beaker to the Erlenmeyer flask (step 5)? Post-Lab Calculations and Analysis 1. Calculate the average pKa value for your unknown weak acid. 2. Comment on the precision (reproducibility) of your pKa determination. Describe sources of experimental error and their likely effect on the measured pKa (pH) values. 3. The following table lists the identities of the unknowns in this experiment. Weak Acid Formula Ka Potassium dihydrogen phosphate KH2PO4 Ka2 of H3PO4 = 6.2 × 10–8 Potassium hydrogen sulfate KHSO4 Ka2 of H2SO4 = 1.0 × 10–2 Potassium hydrogen phthalate KHC8H4O4 Ka2 of H2C8H4O4 = 3.9 × 10-6 Potassium hydrogen tartrate KHC4H4O6 Ka2 of H2C4H4O6 = 4.6 × 10–5 Acetylsalicylic acid 2-CH3CO2C6H4CO2H Ka = 3.2 × 10–4 pKa 4. Compare your experimental pKa value with the literature values reported in Question 3. Determine the probable identity of your unknown. LabKaWeakAcid.docx 3