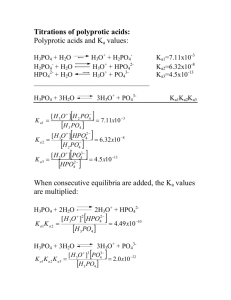
Titration of an Unknown Weak Acid
advertisement

LAB: Titration of a Weak Acid with a Strong Base (DCP, optional CE) In this lab, you will determine which unknown weak acid you are titrating based on the data in the table below. Titrate 100.0 mL (measured with a volumetric flask) of the unknown acid (made by your instructor by measuring out 4.00 g of the solid anhydrous acid and diluting to 1000.0 mL in a volumetric flask) with aqueous 0.10 M NaOH. You will use the PASCO GLX, along with a pH sensor and drop counter, to obtain appropriate measurements during the titration. You will need to calibrate each sensor before use. It may be a good idea to also use an acid-base indicator, such as phenolphthalein, in order to make qualitative observations in addition to quantitative measurements during your lab. Table 1: Unknown Acid Information Name Formula of most common form Glycolic acid HC2H3O3 Equivalent Mass (g/mol) 76.05 Ka pKa 1.6 x 10-4 3.80 Potassium dihydrogen phosphate Potassium hydrogen sulfate KH2PO4 136.09 Ka2 of H3PO4 = 6.2 x 10-8 7.21 KHSO4 136.17 Ka2 of H2SO4 = 1.2 x 10-2 1.92 Potassium hydrogen sulfite KHSO3 120.16 Ka2 of H2SO3 = 6.4 x 10-8 7.19 Sodium dihydrogen phosphate Sodium hydrogen sulfate NaH2PO4H2O 138.01 Ka2 of H3PO4 = 6.2 x 10-8 7.21 NaHSO4H2O 138.08 Ka2 of H2SO4 = 1.2 x 10-2 1.92 Sodium hydrogen sulfite NaHSO3 104.07 Ka2 of H2SO3 = 6.4 x 10-8 7.19 Potassium hydrogen tartrate KHC4H4O6 188.18 Ka2 of H2C4H4O6 = 4.6 x 10-5 4.34 Lab #17