lab: diffusion and osmosis - H
advertisement

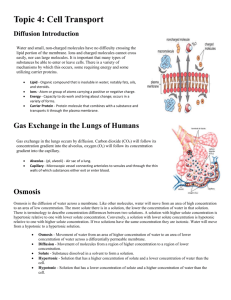
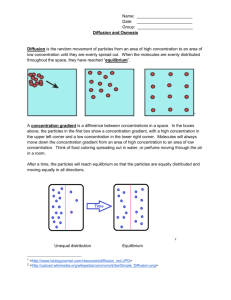
LAB: DIFFUSION, OSMOSIS, & WATER POTENTIAL Demonstration 1: Diffusion. INTRODUCTION At the cellular level, selectively permeable membranes play an important role by isolating and compartmentalizing specific ions and molecules which are necessary to sustain life processes. By regulating the passage of specific molecules into and out of the cell, for example, the plasma membrane is able to create an organized internal environment which is distinctly different from the random distribution of molecules in the external medium. Other membranes, such as those which comprise vesicles, vacuoles, and the ER, serve to further compartmentalize specific subsets of molecules. By altering the number, types, and distribution of imbedded protein channels within these membranes, the permeability, and thus the internal composition of such compartments can be specialized. While permeability is a function of membrane structure, it is equally important to understand the forces that are responsible for moving ions and molecules across such membranes. At any temperature above absolute zero, atoms and molecules possess kinetic energy and are thus constantly in motion. This kinetic energy causes molecules to bump into each other and move in new directions. One result of this molecular motion is the process of diffusion. Diffusion is the random movement of molecules from an area of higher concentration of those molecules to an area of lower concentration. Given enough time, the molecules in question will become evenly distributed within the volume of space they occupy. When a mixture of different molecules undergoes diffusion, each type of molecule diffuses independently of the other types of molecules in the mixture. Diffusion is a passive process; no energy other than their inherent kinetic energy of random motion is required to move the molecules. Osmosis and dialysis are special cases of diffusion. Osmosis is the diffusion of water across a selectively permeable membrane while dialysis is the diffusion of solute particles across a selectively permeable membrane. Because water molecules are extremely small, uncharged particles, they easily diffuse across most membranes. The extent to which dialysis occurs depends on the characteristics (e.g. size, charge) of the solute particles as well as on the nature and quantity of protein channels imbedded within the membrane. Because of their structural organization, cellular membranes also have the ability to move molecules by active transport. Active transport is the movement of molecules across a selectively permeable membrane, typically from an area of lower concentration of those molecules to an area of higher concentration. (Notice that the direction of this molecular movement is opposite that of diffusion). In order to achieve this, energy must be expended by the cell. Such energy is made available from the cellular breakdown of ATP into ADP or by “excited” electrons releasing excess energy as they pass down an electron transport chain imbedded within the membrane. In this demonstration, we will qualitatively measure the diffusion of small molecules selectively in solution through dialysis tubing, an permeable membrane example of a selectively permeable 15% glucose membrane. A solution of glucose and 1% starch starch will be placed inside a bag of distilled water dialysis tubing. Distilled water will be placed in a beaker, outside the dialysis bag. After 30 minutes, the solution inside the dialysis tubing and the solution in the beaker will be tested for glucose and starch. The presence of glucose will be tested with Benedict’s solution: If glucose is present, the Benedict’s solution will turn from transparent blue to opaque orange when heated. The presence of starch will be tested with IKI solution; If starch is present, the IKI solution will turn from transparent yellow to opaque blue/black. Demonstration 2: Osmosis In this demonstration, we will use dialysis tubing to investigate the relationship selectively between solute concentration and the permeable membrane movement of water through a selectively 0.01% starch permeable membrane by the process of osmosis. A solution of 0.01% starch will 1% starch be placed inside a bag of dialysis tubing. The bag will be weighed to the nearest 0.01g and then placed into a beaker containing a solution of 1% starch. After 30 minutes, the dialysis bag will be removed from the beaker, its outside surface blotted dry of excess water, and then reweighed. The change in mass will indicate the direction in which water has moved across the dialysis tubing. When two solutions have the same concentration of solutes, they are said to be isotonic to each other. If these two solutions are separated by a selectively permeable membrane, water will move between the two solutions, but there will be no net change in the amount of water in either solution. If two solutions differ in the concentration of solutes, the solution with more solute is hypertonic to the solution with less solute. Conversely, the solution that has less solute is hypotonic to the solution with more solute. Now consider two solutions separated by a selectively permeable membrane. The solution that is hypertonic to the other must have more solute and therefore less water. Since water moves from an area of high concentration to an area of low concentration, there will be a net movement of water from the hypotonic solution into the hypertonic solution. Botanists use the term “Water Potential” when predicting the movement of water into or out of plant cells. Water potential is abbreviated by the Greek letter psi ( ) and it has two components: physical pressure ( p ) and the effects of solutes ( s ). p Water = Pressure Potential Potential + s Solute Potential Water will always move from an area of higher water potential (i.e. higher free energy; more water molecules) to an area of lower water potential (lower free energy; fewer water molecules). Water potential, then, measures the tendency of water to leave one place in favor of another place. You can picture water diffusing “down” a water potential gradient. lower (more negative) water potential. Generally, an increase in solute potential makes the water potential value more negative and an increase in pressure potential makes the water potential value more positive. Earlier we mentioned that water moves from a region of higher (more positive) water potential to a region of lower (more negative) water potential. Water will continue to move until the water potential of the two regions is equal (if possible). Realize that the if the water potential of a cell does not equal the water potential of its surroundings, then water will enter or leave the cell until the water potential of the cell equals the water potential of its surroundings. The point here is that the cell always adjusts to match the water potential of its surroundings, not vice versa. The following example will help illustrate this point. Example 1: A potato cell (not drawn to scale) is placed in pure water. The cell has an initial solute potential of -3 bars (a bar is a metric measure of pressure, measured with a barometer, that is about the same as 1 atm) and a pressure potential of 1 bars. What will be the water, pressure, and solute potentials of the potato cell at equilibrium? As noted above, water potential is affected by two physical factors. One factor is the addition of solute which lowers the water potential. The other factor is physical pressure. An increase in pressure raises water potential. By convention, the water potential of pure water at atmospheric pressure is defined as being zero ( p s ). Movement of water into and out of a cell is influenced by the solute potential (relative concentration of solute) on either side of the cell membrane. If water moves out of the cell, the cell will shrink. If water moves into an animal cell, the cell will swell and may even burst. In plant cells however, the presence of a cell wall prevents cells from bursting as water enters the cell. In this case, as the incoming water presses the cell membrane against the cell wall, the partially elastic cell wall pushes back, opposing the incoming flow of water. This backwards pressure created by the cell wall is the pressure potential of the cell and it may affect the net movement of water. Pressure potential is nonexistent in animal cells (due to the absence of a cell wall), usually positive in living plant cells, and often negative in dead xylem elements (a negative pressure is referred to as tension and can be thought of as water being “pulled” out of these dead xylem elements). A plant cell in which the cell membrane is not pressed against the cell wall is said to be flaccid and has a pressure potential of zero. It is important for you to be clear about the numerical relationships between water potential and its components, pressure potential and solute potential. The water potential value can be positive, zero, or negative. The pressure potential value can be positive, zero, or negative. The solute potential value is always negative; since pure water has a water potential of zero, any solutes will make the solution have a We see here that the water potential of pure water is, by definition, zero. The potato cell has a solute potential of -3 and a pressure potential of 1 (you are given this information). Since water always moves from a region of higher water potential to a region of lower water potential, water will move from the surroundings ( 0 ) into the potato cell ( 2 ). Water will continue to move into the potato cell until its water potential matches that of the surroundings. When water moves into the cell, the cell membrane will expand and push against the cell wall, increasing the pressure potential. In theory, the incoming water should also dilute the solute concentration of these cells and thus affect the solute potential. However, the incoming water has a much greater influence on the value of pressure potential than on the value of solute potential such that you can ignore any changes which might occur in the value of the solute potential. This assumption is always valid as long as a pressure potential exists. (If pressure potential were zero, then any movement of water into or out of the cell would change the solute potential.) At equilibrium, we know that the water potential of the potato cell must equal zero and we know that the pressure potential is going to increase while the solute potential remains unchanged. For the potato cells then, we essentially have an equation which looks like this: 0 = p + (-3) Solving for p gives us a pressure potential of 3 bars. We have now determined all the values for water, pressure, and solute potentials for the potato cell. When the water potential of the cell equals the water potential of the pure water outside the cell, a dynamic equilibrium is reached and there is no NET water movement. Note that when the equilibrium condition is reached in this example, the solute concentrations inside and outside the cell are not equal. This is because the water potential inside the cell results from the combination of both pressure potential and solute potential. It is the sum of these two components that equals the solute concentration outside the cells. Had this been an animal cell, then the solute concentrations inside and outside the cells would be equal since animal cells do not possess a pressure component. Example 2: Suppose we were to repeat the above experiment at room temperature (25 o C) with a 0.1 M sugar solution instead of distilled water. How would this affect the values of water, pressure, and solute potentials of the potato cell at equilibrium? Again, we would need to determine the water potential of the solution surrounding the cell in order to know whether water will flow into or out of the potato cell in order to reach equilibrium. Since the solution is open to the atmosphere, p 0 . But, since we now have a Take a moment and verify for yourself the values of water, pressure, and solute potentials for the potato cell at equilibrium. THE EXPERIMENT In this experiment, you will obtain and mass six identical samples of plant cells (from two different vegetables). Each sample will be immersed overnight in one of six different sucrose solutions which vary in concentration from 0.0 M to 1.0 M. The following day, each sample of cells will be re-massed and the % gain or loss of water from these cells will be calculated. The results will then be graphed in order to determine and compare the normal water potential of these plant cells. PROCEDURE Each student team will be provided with two vegetables to be compared. sugar solution surrounding the cells instead of distilled water, s 0 . We can calculate the value of s using the following formula: s iCRT where i = C= R= T= Ionization constant (for sucrose this is 1since it does not ionize in water) Molar concentration Pressure constant (R = 0.0831 liter bars/mole K) Temperature in Kelvin (K = 273 + oC of solution) Substituting the appropriate values into the expression, we find s = - (1)(0.1 mole/liter)(0.0831 liter bar/mole K(295K) = - 2.45 bars Therefore, for the surrounding solution, 0 (2.45) 2.45 and the set-up would look like this: Day 1: 1. Using a cork borer and a fresh vegetable, each team will cut five vegetable cylinders of equal length (~5 cm) for each sucrose concentration. Make sure these cylinders are uniform and contain no skin. All five cores collectively represent one vegetable cell sample that will be eventually immersed in one of the sucrose solutions that you decide to use. 2. Obtain six clear plastic cups; label each with your name, group #, period #, and instructor’s name. Into each cup, pour 100 mL of one of the six different sucrose solutions you chose to use. Label the sucrose concentration and initial mass on the cup. 3. Obtain five vegetable cores (i.e. one cell sample), place on a paper towel, and gently role the cores back and forth a few times to remove any excess water adhering to the outside of the cores. 4. Use one of the electronic balances to obtain the initial mass of the cell sample (i.e. all five cores at once). Record this initial mass in you data sheet. Then place the cell sample into one of the labeled sucrose solutions and record the initial mass of these cores on that plastic cup. Let this stand overnight. What were the “normal” water potentials of these two cell types? Animal cells have no pressure potential, but plants in the “normal” state do. If possible, determine the solute concentration (osmolarity) of these two types of cells. If not possible, explain why. b. Day 2: All groups: 1. Using the formula that was provided, calculate the water potential of the sucrose solution(s). 2. Gently retrieve your cell sample from the sucrose solution. Place it on a paper towel; roll it back and forth in order to remove excess water adhering to the outside. 3. Determine the final mass of the cell sample, using the same electronic balance as the previous day. Record this final mass, then discard the cores and plastic cups in the trash. 4. Calculate % gain or loss of water from each cell sample in your group. c. Title 6 Plant final mass initial mass % mass x 100 initial mass 4 2 ANALYSIS 0 -8 1. Now that you have graphed your results for water movement into/out of the vegetable cores, use the x-intercepts to determine the best estimate of the “normal” water potential for these two types of vegetables. For one of the vegetables you used (your choice), at this x-intercept point on the graph, state the relationship among the values of s, p, and . -7 -6 -5 -4 -3 -2 -1 0 -2 Water Potential (Bars) -4 -6 2. During the course of the experiment, which cell type experienced the greatest change in water movement? For this group of cells, separately explain the effect of this inward and outward water movement on the cells’ s, p, and 3. For the potato cells, discuss the following in terms of , s, and p… a. In which direction did water move before equilibrium was reached in the lowest M sucrose solution? Why did this net movement eventually stop? b. Why would there be no change in mass if these cells were immersed in the water potential indicated by the X-intercept? Explain in terms of water movement and equilibrium. 4. Given the graph below (recorded at 200 C)… a. Why was there a positive mass change recorded in the plant cells but no the animal cells? -8 Change in Mass (%) Animal