Chapter 4 Major Classes of Rxns and Titration F11 110
advertisement

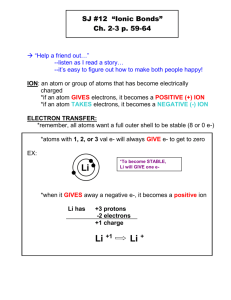
Three Major Classes of Chemical Reactions 4.1 The Role of Water as a Solvent Chapter 4 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions Major Classes of Chemical Reactions 4.4 Acid-Base Reactions 4.5 Oxidation-Reduction (Redox) Reactions 4.6 Elements in Redox Reactions Chapter 4: Reaction Classes Slide 4-1 Determining Moles of Ions in Aqueous Ionic Solutions Electron distribution in molecules of H2 and H2O. How many moles of each ion are in the following solutions? (a) (b) (c) (d) As a result, water is a polar solvent and is able to solvate polar molecules and ionic species 5.0 mol of ammonium sulfate dissolved in water 78.5 g of cesium bromide dissolved in water 7.42 x 1022 formula units of copper(II) nitrate dissolved in water 35 mL of 0.84 M zinc chloride (a) (NH4)2SO4(s) 5.0 mol (NH4)2SO4 x (c) Cu(NO3)2(s) 7.42 x Chapter 4: Reaction Classes Slide 4-2 Chapter 4: Reaction Classes Slide 4-3 1022 2NH4+(aq) + SO42-(aq) 2 mol NH4+ 1 mol (NH4)2SO4 Cu2+(aq) + 2NO3 = 10. mol NH4+ and 5.0 mol SO42- -(aq) mol Cu(NO3)2 formula units x 6.022 x 1023 formula units Cu(NO3)2 = 0.123 mol Cu(NO3)2 Chapter 4: Reaction Classes = 0.246 mol NO32+ and 0.123 mol Cu Slide 4-4 Writing Equations for Aqueous Ionic Reactions Writing Equations for Aqueous Ionic Reactions The molecular equation shows all reactants and products as if they were intact, undissociated compounds. The molecular equation This gives the least information about the species in solution. shows all of the reactants and products as intact, undissociated compounds. 2AgNO3 (aq) + Na2CrO4 (aq) → Ag2CrO4 (s) + 2NaNO3 (aq) The total ionic equation shows all of the soluble ionic substances dissociated into ions. The net ionic equation omits the spectator ions and shows the actual chemical change taking place. When solutions of silver nitrate and sodium chromate mix, a brick-red precipitate of silver chromate forms. Chapter 4: Reaction Classes Slide 4-5 Chapter 4: Reaction Classes Slide 4-6 The total ionic equation shows all soluble ionic substances dissociated into ions. The net ionic equation eliminates the spectator ions and shows only the actual chemical change. This gives the most accurate information about species in solution. 2Ag+ (aq) + 2NO3- (aq) + 2Na+ (aq) + CrO42- (aq) → Ag2CrO4 (s) + 2Na+ (aq) + 2NO3- (aq) 2Ag+ (aq) + CrO42- (aq) → Ag2CrO4 (s) Spectator ions are ions that are not involved in the actual chemical change. Spectator ions appear unchanged on both sides of the total ionic equation. 2Ag+ (aq) + 2NO3- (aq) + 2Na+ (aq) + CrO42- (aq) → Ag2CrO4 (s) + 2Na+ (aq) + 2NO3- (aq) Chapter 4: Reaction Classes Slide 4-7 Slide 4-8 Chapter 4: Reaction Classes An aqueous ionic reaction and the three types of equations. Figure 4.6 The reaction of Pb(NO3)2 and NaI. NaI(aq) + Pb(NO3)2(aq) PbI2(s) + NaNO3(aq) 2NaI(aq) + Pb(NO3)2(aq) PbI2(s) + 2NaNO3(aq) 2Na+(aq) + 2I-(aq) + Pb2+(aq) + 2NO3-(aq) PbI2(s) + 2Na+(aq) + 2NO3-(aq) 2NaI(aq) + Pb(NO3)2(aq) PbI2(s) + 2NaNO3(aq) Double-displacement reaction (metathesis) Chapter 4: Reaction Classes Slide 4-9 Predicting Whether a Precipitate Will Form Chapter 4: Reaction Classes Slide 4-10 Solubility Rules 1. Note the ions present in the reactants. 2. Consider the possible cation-anion combinations (Double Displacement). 3. Decide whether any of the ion combinations is insoluble. To do this we need to know the solubility rules. Chapter 4: Reaction Classes Slide 4-11 Chapter 4: Reaction Classes Slide 4-12 Predicting Precipitation Reactions Acids and Bases: Definitions-1 Predict whether a reaction occurs when each of the following pairs of solutions are mixed. If a reaction does occur, write balanced molecular, total ionic, and net ionic equations, and identify the spectator ions. (a) Potassium fluoride(aq) + strontium nitrate(aq) (b) Ammonium perchlorate(aq) + sodium bromide(aq) (a) KF(aq) + Sr(NO3)2 (aq) 2K+(aq) + 2F-(aq) + Sr2+(aq) + 2KNO3(aq) + SrF2 (s) 2NO3-(aq) (b) NH4ClO4(aq) + NaBr (aq) 2K+(aq) + 2NO3-(aq) + SrF2 (s) NH4Br (aq) + NaClO4(aq) All definitions are operationally equivalent; however the Lewis definition is by far the most general All reactants and products are soluble so no reaction occurs. Chapter 4: Reaction Classes Slide 4-13 14 Chapter 4: Reaction Classes Slide 4-14 The H+ ion as a solvated hydronium ion. Acids and Bases: Definitions-2 “H+”, as a discrete species, does NOT exist in water; rather, it combines with water to give hydronium ion: It is the magnitude of the hydronium ion concentration ([H3O+]; in mol/L) that determines how acidic a solution is! Since H3O+ is generated from H+, and since H+ comes directly from the acid under consideration, measurement of the [H3O+] will have a direct correlation to the strength of the acid! Chapter 4: Reaction Classes Slide 4-15 Chapter 4: Reaction Classes Slide 4-16 Acid-Base Reactions Acids and Bases: Definitions-3 PROTON: an archaic term still used to describe a hydrogen ion (“H+”); in acid/base chemistry it is still used to denote an acidic hydrogen and should NOT be confused with the more traditional definition of a proton (a positively charged nuclear particle). In the most general sense, an acid reacts with a base by transferring an ionizable hydrogen (an “acidic H”) to it: The term “conjugate” is used exclusively to describe the materials on the product side of this reaction. Conjugates are easy to write: PROTON TRANSFER: in acid/base chemistry this is used to denote the transfer of a hydrogen ion from an acid (H-donor) to an acceptor (Hacceptor). If the base is a hydroxide base, a neutralization reaction occurs: WEAK ACID or BASE VS. STRONG ACID or BASE: The strength of an acid or a base is a direct function of the degree of ionization of the acid or base (or, how well it does proton transfer reactions). A relative term. Chapter 4: Reaction Classes H+ interacts strongly with H2O, forming H3O+ in aqueous solution (NOT H+). Slide 4-17 Chapter 4: Reaction Classes Slide 4-18 Acid Strength: Determining the Molarity of H+ Ions in an Aqueous Solution of a Strong Acid Strong Acids: HBr, HCl, HI, HNO3, H2SO4, HClO4 Strong Bases: NaOH, KOH, Ca(OH)2, Sr(OH)2, Ba(OH)2, Group I and Group II oxides Chapter 4: Reaction Classes Sulfuric acid is a major chemical in the fertilizer and explosives industries. In aqueous solution, each molecule dissociates and the H becomes a solvated H+ ion. What is the molarity of H+(aq) in 1.4 M sulfuric acid? Slide 4-19 Slide 4-20 Chapter 4: Reaction Classes An acid-base titration. Writing Ionic Equations for Acid-Base Reactions Write balanced molecular, total ionic, and net ionic equations for each of the following acid-base reactions and identify the spectator ions. (a) Strontium hydroxide(aq) + perchloric acid(aq) (b) Barium hydroxide(aq) + sulfuric acid(aq) Chapter 4: Reaction Classes Start of titration Excess of acid Slide 4-21 Finding the Concentration of Acid from an Acid-Base Titration You perform an acid-base titration to standardize an HCl solution by placing 50.00 mL of HCl in a flask with a few drops of indicator solution. You put 0.1524 M NaOH into the buret, and the initial reading is 0.55 mL. At the end point, the buret reading is 33.87 mL. What is the concentration of the HCl solution? Slight excess of base Slide 4-22 Chapter 4: Reaction Classes SOLUTION: NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l) volume of base = 33.87 mL – 0.55 mL = 33.32 mL 33.32 mL soln x Strategy: Point of neutralization 1L 103 mL x 0.1524 mol NaOH 1 L soln = 5.078x10-3 mol NaOH Since 1 mol of HCl reacts with 1 mol NaOH, the amount of HCl = 5.078x10-3 mol. 5.078x10-3 mol HCl x 103 mL 1 L 50.00 mL Chapter 4: Reaction Classes Slide 4-23 Chapter 4: Reaction Classes = 0.1016 M HCl Slide 4-24 Oxidation-Reduction Reactions The redox process in compound formation. Oxidation: the LOSS (via electron transfer) of one or more electrons: Reduction: the GAIN (via electron transfer) of one or more electrons: Slide 4-25 Chapter 4: Reaction Classes Chapter 4: Reaction Classes Slide 4-26 Oxidation Number (ON) Determination 1. The ON of an atom as an element is zero: Na(s), C(gr), H2(g), I2(s); 2. For a monatomic ion, its charge is its ON; 3. F is always –1; 4. Group I elements are always +1; Group II elements are always +2; 5. Oxygen is usually assigned an ON of –2; the only exception is for peroxides (species where you have O–O bonds) where oxygen has an ON of –1; 6. Hydrogen is +1 when combined with non-metals and –1 when combined with metals (hydrides); 7. The sum of the oxidation states of all atoms in a compound must equal the charge on the compound or ion of interest; 8. Non-integer oxidation states DO exist (Fe3O4) Slide 4-27 Chapter 4: Reaction Classes Oxidation Number (ON) Determination 2 3 For Cr2O7 –2 : [( # chromiums ) ! ( charge of chromium )] + [( # oxygens ) ! ( charge of oxygen )] = charge of Cr2O –27 [( 2x )] + [( 7 ( "2 ))] = –2; solve to get x = +6 Chapter 4: Reaction Classes Slide 4-28 Balancing Redox Reactions: Half Reaction Method • A compound (ionic or covalent) is neutral and has a charge of zero. • An ion WILL have a TOTAL charge; • The sum of all the oxidation states in a compound must equal the overall charge on the ion or molecule. • Set up and solve for the unknown algebraically; For Fe2O3: [( # irons ) ! ( charge of iron )] + [( # oxygens ) ! ( charge of oxygen )] = charge of Fe O [( 2x )] + [( 3( "2 ))] = 0; solve to get x = +3 Chapter 4: Reaction Classes Slide 4-29 1. Write separate equations for both the oxidation and reduction half reactions; 2. Balance under acidic conditions FIRST by doing the following for EACH half reaction separately: a. Balance all elements EXCEPT H and O; b. Balance O using H2O; c. Balance H using H+; d. Balance charge using electrons (e–) 3. Multiply each half reaction by an integer to ensure the number of electrons lost = number of electrons gained**; 4. Add half reactions and cancel species that occur on both the reactant AND product side; 5. Check to ensure balance! Chapter 4: Reaction Classes Slide 4-30 Balancing Redox Reactions: Basic Conditions 1. Balance under acidic conditions FIRST ; get overall balanced equation; 2. Add an equal number of OH–’s as you have H+ present to BOTH sides of the equation; 3. When H+ and OH– are present on the same side, make H2O; 4. Eliminate equal numbers of waters from both sides of the equation until only one side has waters left; 5. Check to ensure balance! Redox Balance: An Example Balance the following: C7 H 8 + MnO 4– ! ! " C7 H 6O 2 + MnO 2 The half-reactions are: C7 H 8 ! ! " C7 H 6O 2 and MnO 4– ! ! " MnO 2 For the carbon species: C7 H 8 ! ! " C7 H 6 O 2 C balanced 2 H 2 O + C7 H 8 ! ! " C7 H 6 O 2 O balanced H balanced 2 H 2 O + C7 H 8 ! ! " C7 H 6 O 2 + 6 H + + 2 H 2 O + C7 H 8 ! ! " C7 H 6 O 2 + 6 H + 6 e Slide 4-31 Chapter 4: Reaction Classes Chapter 4: Reaction Classes Mn balanced 2 H 2O + C7 H 8 + 6 e – + 8 2 H + + 2 MnO 4– ! ! " MnO ! ! " MnO 2 + 2 H 2O O balanced + 4 H + MnO 4– ! ! " MnO 2 + 2 H 2O H balanced charge balanced Multiply the Mn equation by 2 to get correct number of electrons: C7 H 6O 2 + 6 H + + 6 e – + 2 MnO 2 + 4 2 H 2O Final balanced equation: C7 H 8 + 2 H + + 2 MnO 4– ! ! " C7 H 6O 2 + 2 MnO 2 + 2 H 2O in base : C7 H 8 + 2 H + + 2 OH – + 2 MnO 4– !! " C7 H 6 O 2 + 2 MnO 2 + 2 H 2 O+ 2 OH – 2 H 2 O + C7 H 8 ! ! " C7 H 6 O 2 + 6 H + + 6 e – overall : 6 e – + 8 H + + 2 MnO 4– ! ! " 2 MnO 2 + 4 H 2O Chapter 4: Reaction Classes Slide 4-32 Combine reactions and eliminate common species: – 4 3 e – + 4 H + + MnO 4– ! ! " MnO 2 + 2 H 2O charge balanced Redox Balance: An Example Redox Balance: An Example For the manganese species: MnO 4– ! ! " MnO 2 – Slide 4-33 C7 H 8 + 2 MnO 4– !! " C7 H 6O 2 + 2 MnO 2 + 2 OH – Chapter 4: Reaction Classes Slide 4-34 A summary of terminology for oxidation-reduction (redox) reactions. Highest and lowest oxidation numbers of reactive main-group elements. Chapter 4: Reaction Classes Slide 4-35 Chapter 4: Reaction Classes Slide 4-36 Recognizing Oxidizing and Reducing Agents Figure 4.12 An active metal displacing hydrogen from water. Identify the oxidizing agent and reducing agent in each of the following: (a) 2Al(s) + 3H2SO4(aq) (b) PbO(s) + CO(g) (c) 2H2(g) + O2(g) Al2(SO4)3(aq) + 3H2(g) Pb(s) + CO2(g) 2H2O(g) Strategy: Assign an O.N. for each atom and see which atom gained and which atom lost electrons in going from reactants to products. An increase in O.N. means the species was oxidized (and is the reducing agent) and a decrease in O.N. means the species was reduced (is the oxidizing agent). 0 +1 +6 -2 (a) 2Al(s) + 3H2SO4(aq) +3 +6 -2 0 Al2(SO4)3(aq) + 3H2(g) The O.N. of Al increases; Al is oxidized; it is the reducing agent. The O.N. of H decreases; H is reduced; H2SO4 is the oxidizing agent. Chapter 4: Reaction Classes Slide 4-37 Chapter 4: Reaction Classes Slide 4-38 Displacing one metal by another. The activity series of the metals. Chapter 4: Reaction Classes Slide 4-39 End of Chapter 4 Chapter 4: Reaction Classes Slide 4-41 Chapter 4: Reaction Classes Slide 4-40