Colligative Properties Definition: a property of the solvent that
advertisement
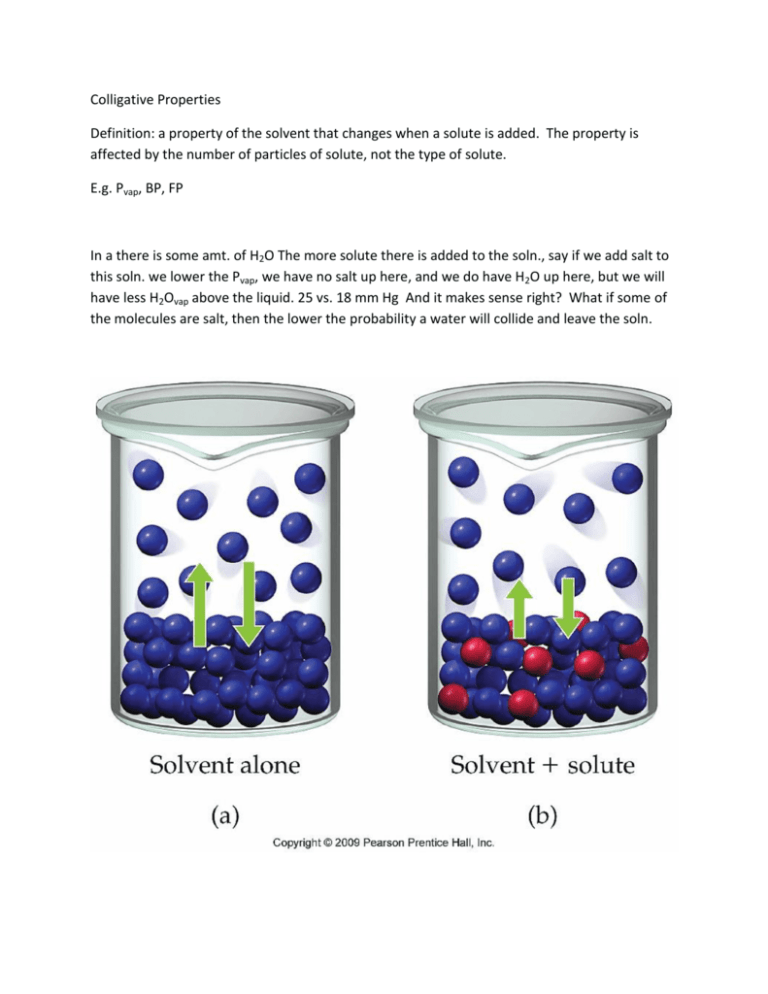
Colligative Properties Definition: a property of the solvent that changes when a solute is added. The property is affected by the number of particles of solute, not the type of solute. E.g. Pvap, BP, FP In a there is some amt. of H2O The more solute there is added to the soln., say if we add salt to this soln. we lower the Pvap, we have no salt up here, and we do have H2O up here, but we will have less H2Ovap above the liquid. 25 vs. 18 mm Hg And it makes sense right? What if some of the molecules are salt, then the lower the probability a water will collide and leave the soln. Psoln = i(Xsolv)( P⁰solv) Raoult’s Law: P⁰ = original Pvap of the pure solvent, X = the mol fraction of the solvent, i = Vant Hoff factor, it doesn’t matter what the solute is, just how many particles of the solute there are. So, lets say I have molecules of glucose, when I dissolve it into water it breaks up into glucose, its Vant Hoff factor is 1. If I put NaCl into the water, it’s VH = 2, meaning for every mole of NaCl I put into water it breaks up into 1 mol of Na+ and 1 mol of Cl-, or 2 pieces, in effect doubling its solvent ability, if I have a mol of CaCl2 my Vant Hoff would be 3. VH = 1 for covalent substances because it doesn’t break into pieces, doesn’t fully ionize The P of the soln (not the P⁰) = should be lower than the original Pvap. Practice: Glycerine (C3H8O3) has a density of 1.26 g/mL at 25⁰ C. Calculate the vapor pressure oa a soln. made by adding 50.0 mLs of glycerine to 500.00 mLs of water. What is the normal Pvap of H2O at 25⁰ C = 23.76 mm Hg P = (1)(X )(23.76 mm Hg) X = mols H2O /mols tot = 500.00 mLs x 1gm/mL x 1 mol/ 18.02 g = 27.7 mols H2O, Mols tot = 50.0 mL x 1.26 g/mL x 1 mol/92.11 g = .684 mols gly So 27.7/27.7 + .684 = .9759 So, I put that back into my equation for P Boiling Point Elevation BP = the temp at which the Pvap = the Patm So, according to Raoult’s Law, if we lower the Pvap, then we will need a higher temp before we get to the correct Pvap Freezing Point Depression: the solute makes it harder for solvent particles to clump particles together. Boiling Point Elevation BP = the temp at which the Pvap = the Patm So, according to Raoult’s Law, if we lower the Pvap, then we will need a higher temp before we get to the correct Pvap Freezing Point Depression: the solute makes it harder for solvent particles to clump particles together. ΔT = iKbm(solute) (Kb = molal bp elevation constant) (for every solvent there is a constant), m = molality of solute E.g. A soln was prepared by dissolving 18.00 g glucose into 150.0 g water. The resulting soln was found to have a bp of 100.34⁰ C . Calculate the molar mass of glucose. Kb = 0.51⁰ C kg/mol ΔT = iKbm(solute) = ΔT = .34⁰ C , I = 1, = .51 .34⁰ C = (1)(.51)(x) X = .34/.51 = .667 molal = .667 molal = x/ .150 kgsolv X = (.667)(.150) = .1000 mols Mol = g/molar mass, .1 = 18/x = 180.18 Freezing Point ΔT = iKfm(solute) = What mass of ethylene glycol (C2H6O2), antifreeze, must be added to 10.0 L of water to produce a soln. that freezes at -10⁰ F, (-23.3⁰ C). Kf H2O = 1.86⁰ C/molal ΔT = iKfm(solute) = 23.3 = (1)(1.86)(x) m = 12.5 molal mol = mol solute/kg 12.5 = x/10 125 mol x 62.08g/mol = 7760 g









