Notebook Quiz chapter 14 Name: What is the difference between a

Notebook Quiz chapter 14 Name:________________________________
1.
What is the difference between a colloid and a suspension?
The particles in a colloid are smaller in size and tend not to settle out.
2.
What is the Tyndall effect and how can it be used to test water for contamination?
The Tyndall effect is the light scattering effect of colloids. If bacterial cells are suspended in water, light will scatter when passed through. The water may appear clear but if it scatters light, it may contain harmful substances that are too small to see.
3.
Describe the difference between the solvation of an ionic substance and a molecular substance.
Ionic substances dissociate (or separate into positive and negative ions), whereas molecular substances do not separate.
4.
How do you create a supersaturated solution?
Supersaturated solutions are created by dissolving as much solute as possible, then heating up the solution, adding more solute and then cooling down the solution to room temperature.
5.
Name two factors that would increase the solubility of a gas in a liquid.
Lowering the temperature and increasing the pressure.
6.
How do solute particles affect the boiling point and freezing point of the solvent?
Solute particles increase the boiling point by holding on to the solvent molecules and decrease the freezing point by separating the solvent molecules.
7.
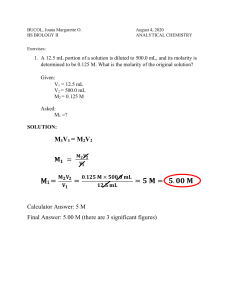
If you dilute 20 mL of a 3.5 M solution to make 100.0mL, what is the molarity of the dilute solution?
M
1
V
1
= M
2
V
2
M
1
= 3.5M
V
1
= 20 mL
V
2
= 100mL
M
2
= ?
M
2
= M
1
V
1
/V
2
= (3.5)(20)/100 = 0.7M
8.
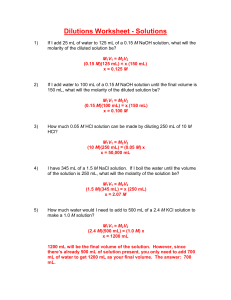
What is the molarity of a 175ml solution that that contains 75g of KCL?
75g KCl X 1 mol X 1000ml = 5.8 M
175 ml 74.5g 1L
9.
How many grams of Na
2
CO
3
must be dissolved in 200 ml of water to create a solution with a molarity of 8.20 mol/L?
8.20 mol X 106g X 1 L X 200ml = 173.84g
1L 1 mol 1000 mL
10.
How many milliliters of 5M phosphoric acid would be needed to make 95 ml of 3M phosphoric acid solution?
M
1
V
1
=M
2
V
2
, V
1
= M
2
V
2
/M
1
V
1
= (3M)(95mL) / 5M = 57 mL