Unit-2 States of matter
advertisement

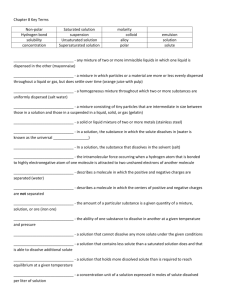
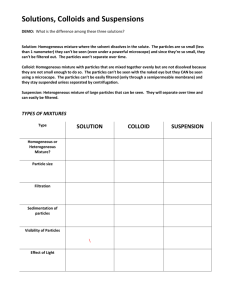
1 Unit‐2 States of matter Matter: It is something that occupies space possessing certain mass. Hence all the Substances present in this universe are matters. Classification of matter on the basis of their physical state: On the basis of physical state, matter can be classified as solid, liquid, gas and plasma. Solid: Matter which possesses definite mass , volume and shape are called solids.In this state particles are held very close to each other.These particles have no freedom of movement. Ex: Wood, stone, copper etc. Liquid: Matter which possesses definite mass and volume but indefinite shape are called liquid. In this state particles are close to each other and they can move within their volume. Ex: water,Benzene,carbon tetrachloride, kerosene. Gas: Matter which possess definite mass but indefinite volume and shape are called gases. In gases particles are far apart as compared to solid and liquid. The particles can move freely from one point to another.Ex: Nitrogen, Oxygen, Carbon dioxide etc. Plasma: It is a highly ionized gas, made up of almost equal number of positive ions and negative electrons. It is formed only at a extremely high temperature of the order of 50000C. It is described as the fourth state of a matter.Plasma is believed tobe the main constituent of stars including sun. Note: The fifth state of matter is called Bose‐Einstein condensate (BEC). This state occur at the end of the temperature scale at a step above absolute zero Kelvin. Classification of matter on the basis of composition: On the basis of the chemical composition matter can be classified as fallows. 1. Heterogeneous matter 2. Homogeneous matter 1) Heterogeneous matter: A substance is heterogeneous if it exhibits different properties at its different position. Different types of heterogeneous matter are a)suspension b) colloid c) heteromixture 2 Matter Heterogeneous matter ______________________ Homogeneous matter ____________________ Suspension colloid Hetero.mixture Homogeneous mixture Pure substance Element ______________________ Metal Non‐metal metalloid _________________ Compound a) Suspension: It is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.These particles can be seen by the naked eye. The solute particles settle down when the suspension is left undisturbed. These can be separated from the mixture by the process of filtration. Ex: dirt particles in water, soar milk. b) Colloid: Colloids are the heterogeneous mixture of two components with the size of the particle is 1nm to 100nm(or 10 A0 to 1000A0).These particles of colloid are uniformly spread throughout the solution. Due to relatively smaller size of the particles as compared to the mixtures ,It appears to be homogeneous, but actually colloids are heterogeneous. These are possessing two phases.(Dispersed phase and dispersion medium) c) Heteromixture: It is obtained by mixing two or more substance in any ratio. These are possessing the mixed properties of the combined substance.These can be separated by physical method. Ex: A mixture of sand and common salt. 3 2) Homogeneous matter: A substance is a homogeneous matter if the smallest part of it exhibits the same chemical and physical properties. Ex: Air , Solution of sugar with water, an intimate mixture of two or more than two metals (alloys). Homogeneous matter can be classified into two types. a) Homogeneous mixtures b) Pure substances a) Homogeneous mixture: A mixture of two components that appears in a single phase is called homogeneous mixtures. These are called as solutions. A homogeneous mixture of solute and solvent are called solutions. These solutions are also called as true solutions or crystalloids Ex:A homogeneous mixture of sugar and water gives true solutions. b) Pure substance: These are made of only one type of particles such as atoms or molecules. Further these are classified as elements and compounds. Element: Simple forms of matter which cannot be decomposed into further simple substances are called elements. 118 elements are discovered till today, out of which 92 are naturally occurring elements and remaining are artificially prepared elements. Ex: Hydrogen, mercury, gold, iron etc. Elements are further classified as metals, non‐metals and metalloids Metal:The elements with the electro‐positive nature of losing one or more electrons readily to give positively charged cations are called metals. M M++ e‐ Ex :Copper,Zinc,Iron Non‐metal: The electronegative elements which have a tendency to gain one or more electrons are called non‐metals. After getting the electrons negative ions formed are called anions. A is a non‐metal and A‐is anion. A + e‐ A‐ Ex: Chlorine, Bromine, sulphur etc . 4 Figure 1 Magnesium metal Figure 2 Sodium metal Metalloids: Elements possessing properties of both metals and non‐metals are called metalloids. Ex: Antimony, arsenic, Germinium etc. Compounds: The substances made of two or more than two elements combined with each other in a definite ratio by mass are called compounds. These can be decomposed into its elements by chemical or electrochemical reactions. Ex: 1. In water(H2O) number of hydrogen and oxygen atoms are in the ratio 2:1 or it contains hydrogen and oxygen combined with each other in the ratio of 1:8 by their mass. i.e. 2g of hydrogen combines with 16 g of oxygen . 2. Sulphuric acid (H2SO4 ):In this compound the ratio of number of atoms of H:S:O combined is 2:1:4. The ratio by mass of H:S:O is 1:16:32.In this compound 2 g of hydrogen 32 g of sulpher 64 grams of oxygen combine with each other to form 98 g of H2SO4. Solution: A homogeneous mixtures of solute and solvent are called solutions. In a solution, a substance which is in a less quantity is solute and other which is in more quantity is solvent. In aqueous solution of sugar, water is solvent and sugar is solute. Concentration of solution. The amount of a solute present in a unit volume of the solution is called concentration of the solution. 5 Concentration of the solution can be expressed in terms such as percentage by mass or by volume, molarity,molality, normality, mole fraction,ppm.Out of these terms the most familiar terms to express the concentration of the solution are percentage by mass or volume, molarity and normality. Percentage by mass: It is the mass of the solute present in 100 g of the solution. Percentage by mass = Percentage by volume: It is the mass of the solute present in 100 cm3of the solution. Percentage by volume = Problem: 50 g of glucose is dissolved in 400 g of water, then calculate % by mass of the solute. Ans: Total mass of the solution = mass of glucose + mass of water = 50 + 400 = 450 g Percentage by mass = = = 11.11 Hence % by mass of glucose in the solution is 11.11 Problem: 300 cm3 of solution contains 20 g of NaCl dissolved with water ,then calculate % by volume of the solution. Ans: Percentage by volume = = = 6.67 Hence % by volume of NaCl in the solution is 6.67 Molarity: Molarity can be defined as the number of gram molecular mass of solute dissolved in one dm3 of the solution. It is denoted by M 6 Molarity = Molarity = or Where m= mass of the solute M= Molecular mass of the solute V= volume of the solution Molarity of NaOH solution is 0.5 M means 0.5 mole ( or0.5 gram molecular mass )of NaOH is dissolved in one dm3 of the solution. Problem.: Calculate the molarity of the solution obtained by dissolving 6 g of oxalic acid in 200 cm3of solution.Mol.mass of oxalic acid is 126. Solution. Given things m=6 g M= 126 V= 200cm3 Molarity = = 0.238M = Normality: Normality can be defined as the number of gram equivalent mass of solute dissolved in one dm3 of the solution. It is represented by N Normality = OR Normality = Where m= mass of the solute E= Equivalent mass of the solute V= volume of the solution Normality of H2SO4 solution is 0.5 N, It means that 0.5 gram equivalent mass of H2SO4 is dissolved in one dm3 of the solution Problem: Calculate the normality of the solution containing 4 g of sodium hydroxide in 500 cm3 of the solution.(Equ.mass of NaOH is 40) Normality = = 0.2 N Hence the normality of NaOH solution is 0.2N 7 Questions 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Define matter Mention the different types of matter on the basis of their physical state. What is a solid ? Give an example. What is a liquid ? Give an example What is a gas? Give an example Write the classification of matter on the basis of their chemical composition. What is a heterogeneous matter ? What is a homogeneous matter? Mention the different types of heterogeneous matter. What is a suspension? Give an example. What is a colloid ? Give an example. Mention the different types of homogeneous matter. Define solution. Give an example for solution. Mention the solute and solvent present in it. What is an element? Give example. What are metals? Give an example. What are non‐metals? Give an example. What are metalloids? Give an example. What are compounds? Give an example. Mention the ratio by mass of hydrogen and chlorine present in HCl. Define the concentration of a solution. Mention the different terms used to express the concentration of the solution. 22 What is % by mass of the solute in solution? Express it in the form of equation. 23 What is percentage by volume of the solution? Express it in the form of equation. 24 Define molarity of a solution. Express it in the form of equation.