Lab: Heating & Cooling Curve
advertisement

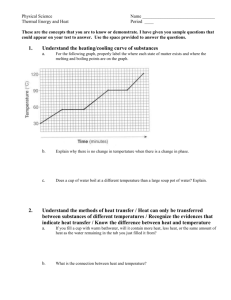
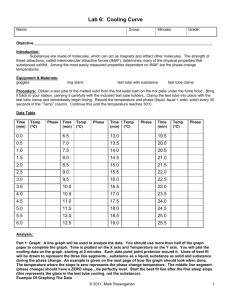
Lab: Heating & Cooling Curve FOR THE TEACHER Summary In this lab, students will create a phase change graph by adding and removing heat to observe and record data during actual phase changes. Instead of just memorizing a heating/cooling curve they see in a textbook, students create their own. Resource Type Lab Submitted by: Tom Flanagan Gonzaga Prep School in Spokane, Washington Thanks to: Ward’s Science Grade Level High school Objectives By the end of this lesson, students will • study the effects of heating and cooling a pure substance through a change of phase • construct heating and cooling curves of a pure substance using experimental data • determine the freezing and melting point temperatures of a pure substance Chemistry Topics This lesson supports students’ understanding of • Phase diagram • Physical change • States of matter • Intermolecular forces Time Teacher Preparation: 30 minutes Lesson: 60 minutes Materials For each group: • • • • • Test tube 18x150-mm containing lauric acid (C12H24O2) Temperature probe Safety goggles Water Hot plate • • • • • Test tube clamp Beaker, 400mL Digital thermometer LabQuest 2 Ring stand Safety • Be careful when using the thermometer to stir the sample. The thermometer is fragile. • Be careful not to allow the cord of the temperature probe to touch the hot plate. • Always wear safety goggles when working in the lab. Teacher Notes This was a new lab that I wrote this year, after consulting many sources. Since the test tubes can be used year after year once initially prepared, and since we have a set of Vernier LabQuest 2 devices, I knew this would be a cost-effective and low waste lab that would fit into a lab period. We used it at both the regular and honors level, and it worked great! FOR THE STUDENT Student Activity Sheet: Heating & Cooling Curve Lab Lesson Background In this experiment, you will observe by direct measurement the effects of cooling and heating a pure substance. In Part A, you will start with a substance in its solid phase at a temperature well below its melting point. While heat is added at a constant rate, temperature readings will be made until the substance is in its liquid phase at a temperature well above its melting point. In Part B, a pure substance will be cooled (heat removed) at a constant rate. Starting with the substance in its liquid phase at a temperature well above its freezing point, temperature readings will be made at regular intervals until the substance changes to its solid phase and cools to a temperature well below its freezing point. The temperature readings will thus show the effects of removing heat from a pure substance in the liquid phase, during a phase change (liquid to solid), and in the solid phase. The data collected in Parts A and B will be used to construct a graph, which will consist of two curved lines: a cooling curve and a heating curve. When completed, the graph will show pictorially what happens to a pure substance as its temperature is raised and lowered over a temperature interval that includes its freezing and melting points. The graph also will show how the freezing and melting points of a pure substance are related. Prelab Questions 1. Write down the terms for the following phase changes (you may need to look them up). I did the first one for you! a. Solid to liquid melting b. Liquid to gas c. Gas to liquid d. Liquid to solid e. Solid to gas f. Gas to solid 2. Of all the phase changes listed above, which ones require heat energy? 3. Which phase changes release heat energy? 4. In which phase do the particles of a substance have the greatest kinetic energy? Purpose To study the effects of heating and cooling a pure substance through a change of phase, to construct heating and cooling curves of a pure substance using experimental data, and to determine the freezing and melting point temperatures of a pure substance. Safety 1. Be careful when using the thermometer to stir the sample. The thermometer is fragile. 2. Be careful not to allow the cord of the temperature probe to touch the hot plate. 3. Always wear safety goggles when working in the lab. Materials test tube 18x150-mm containing lauric acid (C 12 H 24 O 2 ) temperature probe safety goggles hot plate test tube clamp beaker, 400mL digital thermometer LabQuest 2 ring stand water Procedure PART A: HEATING CURVE 1. Set up a ring stand next to a hot plate. Get a 400-mL beaker and fill it threequarters full with tap water. Place it on the hot plate and heat it to a temperature of about 60ºC, using the digital thermometer to measure the temperature. (NOTE: at this point you no longer need to measure the temperature of the water bath. You will now only measure the temperature of the lauric acid). 2. Connect the temperature probe to the LabQuest. Make sure the mode is time based and that the duration is 600 seconds. Start recording. Immerse the test tube of lauric acid below the water level in the hot water bath and clamp it to the ring stand. 3. As soon as the thermometer is free to move, it should be used to gently stir the solid-liquid mixture. 4. Continue stirring and recording the temperature with the LabQuest until the temperature of the sample reaches 50ºC. (NOTE: Be careful when stirring so as NOT to damage the thermometer). 5. Stop the recording of data on the LabQuest, unclamp the test tube from the ring stand, and remove it from the water bath. 6. At this point, save your data file by choosing save from the file and naming your data. PART B: COOLING CURVE 7. Fill a 400-mL beaker three-quarters full with cold tap water. 8. Place the lauric acid back in the hot water bath. Heat the test tube of lauric acid gently until it reaches at least 55ºC. 9. Remove the test tube of lauric acid from the hot water bath. Start recording a new set of data on the LabQuest by choosing new from the file menu and again making sure that the duration is set to 600 seconds. 10. Immerse the test tube into the cold water bath and clamp the test tube of lauric acid to the ring stand. Use the thermometer to gently stir the sample constantly as long as liquid remains. 11. Continue this procedure until the temperature of the sample reaches 30ºC. Remove the test tube from the water bath. Return the tube/probe to the instructor. Results Look at both the heating and cooling curve and determine the melting and freezing points of the lauric acid. Either print or take a picture of both curves. Conclusion How can a substance freeze and melt at the same temperature? It doesn’t seem to make sense, please explain!