Lab – Lauric Acid Cooling and Heating Curve
advertisement

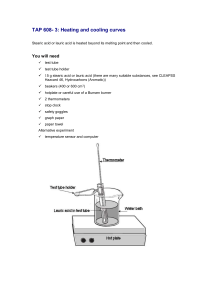
Lab – Lauric Acid Cooling and Heating Curve ‘Cause paradichlorobenzene is just too stinky… What You’ll Be Using • Lauric acid, a solid (at room temperature) fatty acid with the formula C12H22O2. • It’s found in many cosmetic products and soaps/shampoos. What You’ll Be Doing • Because lauric acid is solid at room temperature, you’ll be heating it first. – There’s no way to measure the temperature if you don’t have a thermometer in it. • So the first heating requires no measurement. • We’ll be heating it in a hot water bath. – In other words, attach it to a ring stand with a utility clamp. – The clamp is above the beaker which is on wire mesh on a ring above the Bunsen burner. What You’ll Be Doing • Once the acid liquefies, you’ll remove it from the hot water bath and stick a thermometer in it to record the temperature every 20 seconds until it reaches 40 °C. – If it “needs help” getting that low in temperature, trying using a beaker of room temperature water from the sink. – If it still “needs help” getting that low in temperature, I have an ice bath ready for it. – IMPORTANT: Make sure you can read the thermometer! • At that point, you’ll transfer it to a hot water bath again and record the temperature every twenty seconds as it rises. – HEAT GENTLY FOR QUALITY DATA. What You’ll Be Doing • So think about it: – First you heat it so it becomes a liquid and you can put a thermometer in it. – Then you cool it (and take measurements). – Then you heat it (and take measurements). • At that point you can clean up and start making a graph of the data. – You will need two curves on the same axis. • One for cooling, one for heating. • You’ll also be answering discussion questions. – And speaking of… Pre-Lab Questions • In official chemistry terms, what is the process of going from a solid to a liquid called? What about the reverse? – Fusion (reverse: solidification) • Given the fact that we will be letting a liquid cool into a solid (and vice versa), how many “segments” will each line on your graph have? – Three • Liquid cooling, solidification, solid cooling • Solid warming, fusion, liquid warming Pre-Lab Questions • According to kinetic theory, when you add heat without changing phase, what happens? – Particles move at increasing speed. • When you add heat during a phase change, what happens? – Particles gain energy needed to escape their current state. – Example: When boiling water, when enough heat is added, water molecules escape to the gas state (and move at a faster speed). Quick Safety Notes • Lauric acid isn’t too toxic, relatively speaking. That said, if it gets overheated and aerosolized, it can be a little irritating to the lungs. – Once it’s liquid, you’re ready to go. Don’t overheat. • Use hot mitts! And safety glasses! • When you’re done, put the lauric acid in the ice bath. • Unrelated: – Lauric acid’s real melting/freezing point is…? Lauric Acid Freezing/Melting Point • 43.2 °C.