GuideleinesforLabreportonlauricacid
advertisement

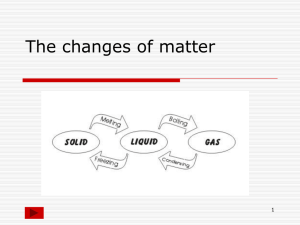
January 2010 Guidelines for Lab Report 10: Cooling Curve of Lauric Acid Your lab report needs to have a title and needs to be organized into the following sections: Title, Introduction, Material and Methods, Results, Discussion, References General Comments: Keep your writing impersonal; use third person past tense. Avoid using I and We Pay attention to grammar and punctuation and divide paragraphs correctly. Some Suggestions for the Introduction: Discuss what matter is. Discuss the various states of matter and how they differ, both in term of volume/mass and their particle arrangement & motion. (In other words, discuss how solids, liquids & gases differ on the macroscopic level and on the molecular/atomic level.) Also discuss the particle attractions in each phase. Discuss phase changes. In your discussion, be sure to explain how & why the particles change during a phase change. Be sure to mention how kinetic & potential energy plays a role. Also, how do attractions between particles change? Also, note which phase changes are exothermic & endothermicexplain these terms as well. Explain what melting point, freezing point, boiling point, and point of condensation are. Explain what a heating and cooling curve show. Make sure to also explain what temperature is. State the objectives or purpose of the experiment. (No hypothesis necessary for this lab) (Make sure to use in-text citations!!) Material and Method: This section is to be written in paragraph form Briefly describe the experimental procedure and equipment that you used. Results: Table 1: Time vs. Temperature table for cooling of lauric acid Table 2: Time vs. Temperature table for heating of lauric acid Graph 1:(must be on graph paper!!!!!) Cooling curve of lauric acid. Graph 2: Heating curve of lauric acid. ** Be sure to appropriately title and label all axes, and be sure to spread your graph out so that the trend can easily be seen.** Some suggestions for the Discussion Include an interpretation of your results. Discuss what happened to the lauric acid as you moved it from the hot bath to the cool bath. Discuss the trend you see in your graph. (For example, why do you see your curve leveling off at one point but then increasing/decreasing at another?) Explain the graph using your knowledge of states of matter/phase changes, as well as your knowledge of what is going on a particle-level during phase changes. (**Here, you are connecting the theory from your introduction to your results!**) Based on the cooling curve, what might the freezing point of lauric acid be? Based on the heating curve, what might the melting point of lauric acid be? Explain what on the graph made you come to that conclusion. Compare the two values (melting point & freezing point). Should they be the same or different? The freezing/melting point of lauric acid is about 42 degrees Celcius. Compare to what your lab data states. Why might your data be different? Discuss potential experimental errors. Based on your data, what conclusions might you make about the relationship between the boiling point and point of condensation of a substance? What conclusions can you make about what happens to the temperature of a substance as it boils or condenses? Why does this happen? Were the objectives of the experiment met? Recommendations for further experiments? References List all your resources (books used, data tables, web sites consulted- only .gov or .edu sites are acceptable) that you used in writing the introduction and discussion and cite your references using the APA guidelines. Remember, a minimum of 3 references is required.