Analysis of Mystery Solutions Experiment
advertisement

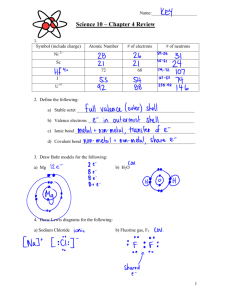
Name: _____________________________________ Period: _______ Date: ____________________________ Analysis of Mystery Solutions Experiment A Double Replacement Investigation Purpose: To identify the contents of six unknown solutions and look for solubility patterns Materials: 24-well spot plate, 0.1 M solutions of the following: silver nitrate, potassium iodide, sodium chloride, lead (II) nitrate, 0.5 M sodium carbonate, and 1.0 M hydrochloric acid. Procedure: 1. Add 2 drops of silver nitrate solution to the well marked #1 on the spot plate. 2. To the same well, add 2 drops of hydrochloric acid solution. Record any observations to the Data Table. 3. In a similar manner, mix each of the other solutions listed in the Data Table. After each test, record in the appropriate box what was formed and what you saw. Carefully describe any products that were formed. If nothing happened, write “No Reactions”. Data Table: Silver Nitrate AgNO3 Hydrochloric Acid HCl Sodium Chloride NaCl Silver Nitrate AgNO3 Hydrochloric Acid HCl Sodium Chloride NaCl Potassium Iodide KI Lead (II) Nitrate Pb(NO3)2 Sodium Carbonate Na2CO3 Honors Chemistry Potassium Iodide KI Lead (II) Nitrate Pb(NO3)2 Sodium Carbonate Na2CO3 Name: _____________________________________ Period: _______ Date: ____________________________ Testing the Mystery Solutions 1. Gather a set of six bottles containing the six mystery solutions 2. Record the number of your set of bottles. Number of Unknown Set _______________ 3. Rinse and dry your spot plate. Begin testing as before by choosing two unknown solutions and mixing together two drops of each. Continue in this manner until all the solutions have been mixed. 4. Keep a careful record of each reaction resulting from the mystery solution pairs. A B C D E F A B C D E F Analysis & Conclusions 1. State the identity of each unknown solution 2. Write word equations and balanced chemical equations for each reaction that occurred. Be sure to include the phase of the reactants and products. 3. What general conclusions may be drawn about the solubilities of compounds containing silver, sodium, potassium, and lead? Write a paragraph discussing any observed trends. Honors Chemistry