Worksheet 21
advertisement

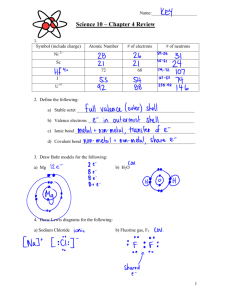
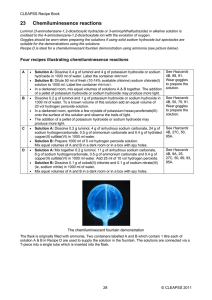
Worksheet 21 Directions: write balanced net ionic equation for the following. Answer the associated question 1. Solutions of sodium hydroxide and lead (II) nitrate are mixed If 1.0L volumes of 1.0 M solutions of sodium hydroxide and lead (II) nitrate are mixed how many moles of product(s) will form. Assume reaction goes to completion 2. Excess nitric acid is added to solid calcium carbonate Briefly explain why marble statues (calcium carbonate) displayed outdoors in urban areas are deteriorating 3. A solution containing silver (I) ion (oxidizing agent) is mixed with a solution containing iron (II) ion (reducing agent) If the contents of the reaction mixture described above are filtered, what substance(s), if any, would remain on the filter paper? 4. Solid ammonium carbonate decomposes when heated Draw the lewis structure(s) for the carbonate ion 5. Chlorine gas, an oxidizing agent, is bubbled through a solution of potassium iodide What is the oxidation number of chlorine before and after the reaction takes place 6. A small piece of sodium is placed in a beaker of distilled water What product of the reaction would burn to produce flames? 7. Aqueous solution of sodium hydroxide is added to a saturated solution of aluminum hydroxide forming a complex ion If the resulting mixture is acidified would the concentration of the complex ion increase, decrease, or remain the same? Explain 8. Phosphorous trihydride is oxidized by oxygen gas If three moles of phosphorous trihydride mix with three moles of oxygen, which reactant any will be in excess? Explain 9. Solid potassium oxide is added to water If a few drops of phenolphthalein are added to the resulting solution, what would be observed? Explain. 10. Ethane combusts in the presence of oxygen Predict the sign of ΔH. Justify your answer 11. A sample of solid iron (II) oxide is completely reduced with solid carbon What is the oxidation number of carbon before and after the reaction? 12. Equal volumes of equal molar solutions of ammonia and hydrochloric acid are combined Is the resulting solution is acidic, basic, or neutral. Explain 13. Solid mercury (II) oxide decomposes as it is heated in an open test tube in a fume hood. After the reaction is complete, is the mass of the material in the test tube greater than, less than, or equal to the mass of the original sample? Explain. 14. Solutions of barium nitrate and potassium fluoride are mixed forming a precipitate If equal molar amounts of reactants are combined, which reactant is the limiting reactant? Explain 15. A piece of cadmium metal is oxidized by adding it to a solution of copper (II) nitrate. List two visible changes that would occur during the course of the reaction