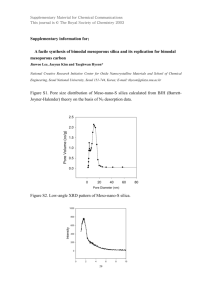
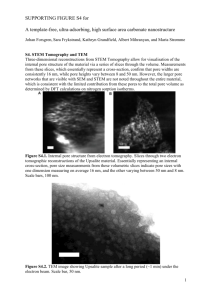
Design of mesoporous silica templates for nanoparticle growth
advertisement

Thesis for the degree of Master of Science D ESIGN OF M ESOPOROUS S ILICA T EMPLATES FOR NANOPARTICLE G ROWTH Emma Johansson Supervisor Magnus Odén Examiner Magnus Karlsteen Department of Physics GÖTEBORG UNIVERSITY Design of mesoporous silica templates for nanoparticle growth EMMA JOHANSSON c 2008 EMMA JOHANSSON Copyright ° Göteborg University Department of Physics SE-412 96 Göteborg Sweden Telephone: +46 (0)31 773 10 00 Web: http://physics.gu.se Abstract The synthesis conditions for making mesoporous silica, SBA-15, have been studied. The purpose of the study was to control and increase the pore size and structure of SBA-15. Methanol, Ethanol, Isopropanol, Formaldehyde, Acetone and THF have been used as swelling agents and the reaction temperature, pH, hydrothermal treatment time and temperature have been varied. The products were characterized using physisorption, SEM, TEM and XRD. The study showed that all swelling except THF are working as swelling agents when the swelling agent/P123 ratio is 210 but when it was increased to 420, the order was lost. It was further showed that when the reaction temperature is varied from 35 to 50◦ C and ethanol is used the order in the material goes from nonordered to completely ordered. The study also shown that when methanol is used as a swelling agent and the pH is increased to 1 or 2, the reaction goes much slower and the resulting products are snapshots of the formation process. It was also shown that in opposite from reports in literature for samples with or without swelling agents, there is no expansion of the pore size when the hydrothermal treatment time is increased for samples with alcohols used as swelling agents. When the hydrothermal treatment temperature is increased the pore size is though increased even for these samples. iii iv Acknowledgement I thank my supervisor Professor Magnus Odén for the opportunity of doing this project and for all his support and ideas. Thank you for believing in me and giving me freedom to explore this area. Also, I thank my examiner Associate Professor Magnus Karlsteen for all his support and patients during all of my study years. Without you everything would be different. Ph. D. José Manuel Cordoba Gallego has my greatest gratitude for all his knowledge, many hours with the TEM and infinite discussions. Without your support and guidance I would have destroyed more than one lab coat... Furthermore, I thank Thomas Lingefelt and Kalle Brolin for all their help with lab equipments the nitrogen tank. It has made my life much easier. I also thank Hung-Hsun Lee for all her help with the FT-IR. Of all my friends, I will especially thank Lina Rogström who convinced me to move to Linköping and is a true friend. I also thank all my new friends and colleagues at IFM. You are great and make my life richer. Finally, I thank Jonas and my family for their love and support. You are always encouraging and listen to my stories even when you have no idea of what I am doing. v vi Contents 1 Introduction 1 2 Mesoporous silica, SBA-15 2.1 Mesoporous silica . . . . . . . . . . . . . . . . 2.2 Structure . . . . . . . . . . . . . . . . . . . . . 2.2.1 Pore structure . . . . . . . . . . . . . . 2.2.2 Corona . . . . . . . . . . . . . . . . . 2.2.3 Formation . . . . . . . . . . . . . . . . 2.2.4 Phase transitions . . . . . . . . . . . . 2.3 Reagents . . . . . . . . . . . . . . . . . . . . . 2.3.1 Surfactant . . . . . . . . . . . . . . . . 2.3.2 Silica precursor . . . . . . . . . . . . . 2.4 Pore control . . . . . . . . . . . . . . . . . . . 2.4.1 Micelle control . . . . . . . . . . . . . 2.4.2 Swelling agents . . . . . . . . . . . . . 2.4.3 Additional/alternative silica precursors 2.4.4 Salts . . . . . . . . . . . . . . . . . . . 2.4.5 Reaction conditions . . . . . . . . . . . 2.4.6 Hydrothermal treatment . . . . . . . . 2.5 Summary . . . . . . . . . . . . . . . . . . . . 3 Experimental design 3.1 Synthesis . . . . . . . . . . . 3.1.1 Samples . . . . . . . . 3.1.2 Additives . . . . . . . 3.2 X-Ray Diffraction . . . . . . . 3.3 Physisorption . . . . . . . . . 3.3.1 Adsorption isotherms . 3.3.2 BET Surface Area . . 3.3.3 BJH Pore Volume . . . 3.3.4 KJS pore size analysis . . . . . . . . . vii . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 3 4 4 5 6 9 9 10 11 11 11 11 13 14 15 16 18 . . . . . . . . . 21 21 21 22 23 24 25 25 26 27 CONTENTS viii 3.4 3.5 3.6 Scanning electron microscopy . . . . . . . . . . . . . . . . . . . Transmission electron microscopy . . . . . . . . . . . . . . . . . Fourier transformed infrared spectroscopy . . . . . . . . . . . . . 28 29 29 4 Results 4.1 Original sample . . . . . . . . . . . . . . . . . . . . . . . 4.2 Swelling agents . . . . . . . . . . . . . . . . . . . . . . . 4.2.1 Different additives . . . . . . . . . . . . . . . . . 4.2.2 Increasing the amount of additives . . . . . . . . . 4.2.3 Hydrothermal treatment of samples with additives 4.3 Reaction temperature . . . . . . . . . . . . . . . . . . . . 4.4 pH . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.5 Calcination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 31 33 33 37 39 44 48 50 5 Discussion 5.1 Original sample . . . . . . . . . . . . . . 5.2 Swelling agents . . . . . . . . . . . . . . 5.2.1 Different additives . . . . . . . . 5.2.2 Increasing the amount of additives 5.2.3 Hydrothermal treatment . . . . . 5.3 Reaction temperature . . . . . . . . . . . 5.4 Changing pH . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51 51 51 51 54 55 58 59 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 Conclusions 61 7 Future works 63 Bibliography 64 A Samples 71 Chapter 1 Introduction Mesoporous materials are materials with a pore diameter of 2-50 nm. These materials are of great interest due to their large surface area which makes them suitable as catalysts. They are also used as templates for making nanoparticles, chemical sensors and as molecular sieves. The synthesis of these materials are of great interest, since small changes in the procedure can result in large changes in morphology, pore structure and size of the mesopores. In this project, the synthesis conditions for making mesoporous silica, SBA-15 where SBA stands for Santa Barbara Amorphous, have been studied. The aim of the study is to control and increase the pore size of SBA-15. The applications of SBA-15 are many. It is used as a template both for making nanoparticles and for making other mesoporous structures. Other applications are as mentioned above catalysts, but another idea is to use it as a drug delivery system. In all applications when the thought is to put something inside a pore, the size of this pore is crucial. When making nanoparticles, the maximum size of the particle will naturally be the size of the pore. For separating molecules it is also obvious that the pores must be larger than the material one will separate and smaller than the molecules one will separate from. In drug delivery, the drugs are often very large molecules and demands therefore large pores in the surrounding material. It is therefore a need for controlling and increasing the pore size of SBA-15, for which the maximum pore size now is 15 nm. In this study we have investigated the possibilities of changing the pore size of SBA-15 by adding swelling agents, changing the reaction temperature, pH and parameters for hydrothermal treatment. 1 2 CHAPTER 1. INTRODUCTION Chapter 2 Mesoporous silica, SBA-15 This chapter contains a literature study about mesoporous silicas and especially about SBA-15 which is the material that has been produced in this diploma work. First a short overview of mesoporous silicas will be presented, followed by a more detailed explanation of the formation of SBA-15 and how other researchers have tried to tune the properties of the material. 2.1 Mesoporous silica In 1992 a new family of mesoporous materials, M41S, was discovered [1, 2]. The material possessed regular arrays of uniform channels which diameter could be tailored through the choice of surfactant and reaction conditions. This was the start of a new era in material science. Mesoporous materials have a pore size of 2–50 nm [3], where one with pore size refers to the diameter of the channels [4]. The mesoporous materials are suitable for catalysis and adsorption due to the high surface area of the materials. The properties of M41S materials are easy to tune as mentioned above. The materials are often referred to MCM materials, which stands for Mobil Composition of Matter. The most popular MCM materials are MCM-41 and MCM-48 which have a hexagonal packing of the pores and a cubic mesophase respectively [5]. The first mesoporous silica made with amphiphilic triblock copolymers were reported in 1998 [6, 7]. These materials are called SBA-X where SBA stands for Santa Barbara Amorphous and X represents a number so that e.g. SBA-15 represents a 2-dimensional hexagonal structure, SBA-12 a 3-dimensional hexagonal structure and SBA-11 has a cubic structure. SBA-15 is a very popular material since it is possible to make during various 3 4 CHAPTER 2. MESOPOROUS SILICA, SBA-15 conditions and it is quite easy to control the pore size with the use of swelling agents such as tetramethylbenzidine, TMB. In the first study of SBA-15 [7] it was reported that it is possible to form pores in the range 46–300 Å. This is unfortunately not completely true since the material undergoes a phase transition when to much TMB is used [8], forming a mesocellular foam, MCF, when the pore size of SBA-15 exceeds 12 nm due to too much TMB. In commercial use there are large requirements on the synthesis process. The process should be of low cost, easy to make small or large batches and effective and not a hazard to the environment or employees. SBA-15 is a product that is not very expensive and it is easy to change the batch size. The material has many applications such as catalysts, drug delivery system [9], as hard templates for nanocasting of oxide nanoparticles [10] and also as a template for making mesoporous replicas [11]. Mesoporous materials can be used for thin films, but one problem is that the pores are along the substrate and not accessible from the surface [12] 2.2 Structure In SBA-15, the mesopores are cylindrical and organised in a hexagonal lattice. Between the cylindrical pores, there are micropores which connect the cylinders to each other. The size of the mesopores in SBA-15 varies between 6.5–15 nm and the thickness of the pore walls range from 3.1 to 4.8 nm. 2.2.1 Pore structure The first study of the wall structure of SBA-15 was performed by Ryoo et al in 2000 [13]. A simple calculation of the relation between the structural parameters pore width, w, surface area, S, and pore volume V shows that for a pure cylindrical geometry have the relation wS = 4. (2.1) V This is not the case for SBA-15 for which this relation always is larger. This has been explained by micropores in the walls of the cylindrical mesopores, giving a higher specific surface area. These smaller pores are disordered and nonuniform since they do not give any peaks in the XRD. From calculations of the nitrogen isotherms it is possible to estimate the microporosity of materials. In this case, the material showed a broad distribution of small pores with a maximum at around 2 nm. 2.2. STRUCTURE 5 The third thing in this study that shows the presence of micropores in SBA-15 is the platinum replica. The pores were filled with platinum and then the silica walls were dissolved with hydrofluoric acid. The replica showed bundles of parallel wires, where the wires have the same diameter as the pores in the silica. The same experiment was performed using MCM-41 and in this case there were also wires formed but they were not arranged in bundles with a fixed distance as in the case with SBA-15. Figure 2.1: Structure of SBA-15. This proves that inside the walls of SBA-15 there are networks of smaller pores, connecting the mesopores to each other. A systematic image of this structure are seen in figure 2.1 2.2.2 Corona The micropores around each mesopore are called the corona. The corona has been studied by Impéror-Clerc et al [14] using XRD. They saw that around each pore there is a volume with lower density than in the pure silica. This change in density is explained by the hydrophilic tales of the surfactant that gets stuck in the silica walls prior to calcination. Ruthstein et al [15] studied the origin of the micropores in SBA-15 using EPR spectroscopy. They showed that the microporosity is due to the EO chains that are trapped in the silica network. This is illustrated in figure 2.2. In the final product, the majority of the EO chains are located in the micropores. The amount of microporosity is according to Ruthstein et al depending on the hydrothermal treatment temperature and the Si/P123 ratio. If the Si/P123 ratio is increased, the thickness of the silica network increases and this yields a larger relative amount of micropores. Another model for the microporosity in SBA-15 is suggested by Vradman et al [16]. In this model it is proposed that the micropores are stress-induced defects in solid pore walls due to mechanical forces during the self-assembly and postsynthesis steps. The idea is the same as for macroscopic materials: the larger an 6 CHAPTER 2. MESOPOROUS SILICA, SBA-15 object is the more defects it accumulates during preparation. If this is true even for mesoporous silica the amount of microporosity should increase with the thickness of the pore walls. MCM-41 does not have any micropores, but this is correct according to the model since the pore walls in MCM-41 is very thin. Figure 2.2: Model for the SBA-15 structure before the thermal stage. The light grey colour represents the silica network, the dark grey corresponds to the part of the EO chains in the mesopores and the white colour is the PO chains in the mesopores. The black dots are the NO groups of L62-NO spin label [15]. One way to affect the corona is by hydrothermal treatment. Impéror-Clerc et al [14] showed that the unit cell of the hydrothermal treated sample decreases by only 3-8% during calcination. This can be compared with shrinkage of 20% for the untreated sample. Also the corona decreases during calcination for untreated samples while this is not the case for the hydrothermaly treated ones. In their study it was shown that the density of the corona is higher for hydrothermal treated samples and that there is no microporosity in these samples. Also the treated samples have a larger pore diameter than untreated samples. More about this can be read in section 2.4.6. 2.2.3 Formation There are four main steps in the preparation of SBA-15. The first step is the synthesis of the mesoporous structure using a block copolymer, in our case P123, and a silica precursor, in our study TEOS. The second step is the hydrothermal treatment at a higher temperature. Filtration and washing the samples are the third step and the fourth step is removing the polymer by calcination. A schematic of the 2.2. STRUCTURE 7 formation process is seen in figure 2.3. In the synthesis the surfactants are added to a homogeneous, acidic solution containing distilled water and HCl. In some cases swelling agents are added to the solution before adding the surfactant. For SBA-15 the triblock copolymer P123 is used as the surfactant. The P123 forms spherical micelles in the solution. When the silica precursor is added it hydrolyzes and bond to the micelle forming a silica network that will be the walls in the particles. At this step the micelles becomes elongated from spheres to cylinders. Several studies of this formation have been performed. Figure 2.3: Possible synthesis pathway for the synthesis of hexagonal silica [2]. The reaction mechanism can be viewed at three length scales: the molecular one, the mesoscopic scale and the macro scale. In these scales one can study the interactions between the molecules, the development of micellar structures and the onset of long range order and the morphology of the particles respectively. This section will deal with the molecular and mesoscopic scales. Ruthstein et al [17] studied the early stages of the formation of micelles, where the interaction between silica precursors and surfactants occurs. They showed that when the silica precursor, in their case TMOS, is added to the micellar solution the interaction has four stages. In the first stage, the TMOS and partially hydrolyzed TMOS penetrate into the core of the micelles. This is due to the hydrophobic properties of the TMOS. In the core, the hydrolysis of TMOS occurs rapidly and the hydrolyzed monomers diffuse into the corona region. There, the polymerization process of the silica begins. During the first hour after adding TMOS the water from the corona/core interface region is removed and the silica forms a network outward. During the third stage this process proceeds in the corona. In the final stage there is a continuous increase of the core and a decrease in the silica wall thickness. An illustration from this article is seen in figure 2.4. This can be compared with a study by Flodström et al [18], who used NMR and 8 CHAPTER 2. MESOPOROUS SILICA, SBA-15 TEM to study the formation mechanism. In this study one can see the four stages in the formation. In the first stage there are still spherical micelles. These are in the second stage elongated, forming cylindrical micelles. In the third stage the micelles starts to pack in a hexagonal structure and in the final stage there is a clear hexagonal structure. These results are also confirmed by a XRD-study made by Flodstrom et al [19]. Figure 2.4: The formation process according to Ruthstein et al [17]. The dark grey illustrates a large amount of water in the corona region. The light grey illustrates a region with less water. A study using cryo-TEM was performed by Ruthestein et al [20] which showed the formation of micelles in a solution and the following steps until the hexagonal packing is formed. The steps followed as in the earlier mentioned studies. After the addition of silica precursor the spherical micelles transformed into elongated micelles which further developed into long threadlike micelles. During this growth time the polarity and water content of the core/corona interface decreases which changes the packing number of the surfactant and leads to a less curved surface of the micelle. The micelles become less flexible and straighter with the condensation of silica in the corona. The number of these flocks increases which leads to precipitation and the hexagonal structure will later be formed. Another interesting observation in their study is that there are always two phases present in the solution after adding the silica precursor. One is the more silica rich one in which the elongated micelles are formed and a second, more silica lean one which consists of spherical micelles. This is also seen in all samples that have been produced in our project. 2.3. REAGENTS 9 The transition from spherical to cylindrical micelles can also be seen from another angle. Before the addition of silica precursors the hydrophilic heads of the P123 are close to the Cl− ions in the solution. The chloride ions will repel the hydrophilic heads and thereby increase the packing factor [8] which is defined by the ratio of the area of the tail, aT , and the area of the head, aH , aT P = . (2.2) aH Depending on the packing factor, the micelles will have different shapes. For P<1/3, spheres will be formed. When 1/3<P<1/2, cylinders are formed and for P>1/2 there will be a lamellar structure. When the silica precursor then is added, the precursors will go into the core of the micelles and thereby increase the area of the hydrophobic tails. At the same time, the silica in the corona will screen the repulsion between the hydrophilic heads and the Cl− , decreasing the area of the head. These two effects together increase the packing number and there will be a phase transition from spherical to cylindrical micelles. 2.2.4 Phase transitions The pore size in SBA-15 can be controlled in a series of ways. To our knowledge, the largest SBA-15 organised pore sizes reported are ca 15-16 nm [21, 22]. Experiments with TMB as a swelling agent have been performed [7], but a large amount of TMB will lead to a phase transition from an ordered hexagonal structure to a mesocellular foam (MCF) [8]. The foam has interconnected, spherical pores with diameters of 220-420 Å. A study performed by Schmidt-Winkel et al. [23] showed that these foams contain uniformly sized spherical cells that are interconnected by uniform windows. The hysteresis loop from nitrogen sorption data reveals that the foam possess inkbottle-type pores, by which means that there are large pores connected by narrow windows. The pore size in MCM-41 is usually 20-30 Å. By using a swelling agent as TMB the pore size of MCM-41 can be expanded to 100 Å [7]. 2.3 Reagents In the synthesis of SBA-15 the following ingredients are used: distilled water, hydrochloride acid (HCl), a surfactant and a silica precursor. 10 CHAPTER 2. MESOPOROUS SILICA, SBA-15 2.3.1 Surfactant The surfactant used for the formation of SBA-15 is P123 which is a triblock PEOPPO-PEO copolymer. The structure of this molecule is seen in figure 2.5. P123 is an amphiphilic molecule where the PEO parts are hydrophilic and PPO part is hydrophobic. The PEO parts are in this work referred to as EO chains and PPO as PO chain. The amphiphilic behaviour of P123 makes it form spherical micelles in water with the EO chains towards the water and the PO chains in the core of the micelles. Figure 2.5: Structure of P123. To make the surfactants forming micelles there must be a minimum number of them in the solution. This is called the critical micelle concentration, CMC. The CMC is temperature dependent and for P123 the CMC is 0.4 g/L at 22◦ C and 0.04 g/L at 30◦ C [24]. It is important to know that a higher amount of surfactants than the critical micelle concentration will only form more and no larger micelles [25]. The concentration and EO/PO ratio of the polymers are very important. A EO/PO ratio above 1.5 tends to favour the formation of cubic mesoporous silica structure while a lower ratio at 0.07-1.5 will favour a hexagonal structure. If the ratio is less than 0.07 and the concentration is low, 0.5-1 wt%, a hexagonal SBA-15 structure will be formed but if it is higher it will form a lamellar mesostructured silica [6]. The dependence between the EO chain length and the obtained mesophase has been shown by Kipkemboi et al [26]. If the EO chain is short, 4 units, the structure is lamellar. 13-37 units give a hexagonal structure and long chains, 132 units, a cubic structure. It also shown that the pore size is determined by the size of the PO chain and that the length of the EO chain is not important. Combining these results with the one from Zhao et al [6] it is obvious that the length of the EO chain is crucial for which structure the pores will form, but it is not possible to say if this is the only truth or if it is the EO/PO ratio that determines the pore structure. The effects of additives, pH and reaction temperature on the micelle formation and behaviour will be further discussed in section 2.4. 2.4. PORE CONTROL 2.3.2 11 Silica precursor In this study two Tetraethyl oxysilane (TEOS) was used as silica precursor. This molecule is in the group of tetra-alkoxysilanes and its structure is seen in figure 2.6. Figure 2.6: Structure of TEOS. The hydrolysis of TEOS [27] can be seen as Si (OC2 H5 )4 + H2 O → Si(OH)4 + 4C2 H5 OH (2.3) Si(OH)4 → SiO2 + 2H2 O. (2.4) The rate of hydrolysis of the tetra-alkoxysilanes depends on the environment. Variables such as temperature, amount of ions and also the type of solvent are important for the rate of hydrolisation of the precursors. This will further be discussed in section 2.4, Pore control. 2.4 Pore control 2.4.1 Micelle control During the formation of the micelles, the temperature is a factor that directly affects among other things the micelle radius and critical micelle concentration. Yamada et al. [28] showed that the radius of the micelles depends on the core size of the micelle. This radius is determined by the solution temperature and therefore should this temperature be an effective parameter for controlling the pore size. The relation between the core radius of the triblock copolymers and the solution temperature is R ∼ (T − TC )0.2 (2.5) where TC is the critical micellization temperature. 2.4.2 Swelling agents A swelling agent is a molecule that goes into the micelles and expands it. Known swelling agents for SBA-15 is TMB, ethanol and alkenes. 12 CHAPTER 2. MESOPOROUS SILICA, SBA-15 TMB One of the most frequently used swelling agents is tetramethyl benzidine, TMB. At first it was thought that TMB could expand the pore size of SBA-15 to 30 nm [7] but later it was shown that a large amount of TMB makes the silica transform from SBA-15 to MCF [8]. Still, small amounts TMB acts as a good swelling agent for mesoporous silicas and is commonly used for making SBA-15 with large pores. One of the benefits with using a swelling agent such as TMB instead of expanding the pores with hydrothermal treatment is that the thickness of the silica walls do not decreases with increasing pore size. Alkenes One of few studies that has obtained highly ordered SBA-15 with pores larger than 12 nm was performed by Sun et al. [22]. In this study, alkenes such as hexane, heptane and nonane were used as swelling agents. By careful control of the reaction temperature and an addition of NH4 F SBA-15 with a pore size of 15 nm was obtained. It was shown that shorter alkenes need a lower reaction temperature but give larger pores. The reaction temperature in this study spans from 10◦ C to a specific temperature for each alkane, e.g. 23◦ C for heptane. Above this temperature structures like MCF will form and below 10◦ C the product will be an amorphous silica gel. The hexane recipe used in the study of Sun et al was repeated by Kruk et al [29]. They confirmed that it is possible to produce large pore SBA-15 during this low temperature synthesis. They also showed that it is possible to increase the pore size further by increasing the hydrothermal treatment time and/or temperature. Another study of the effect of alkenes as swelling agents were made by Blin and Su [30]. In this study the usual route for making SBA-15 was not used. Instead cetyltimethylammonium bromide, CTMABr, was used as the surfactant and sodium silicate instead of TEOS. This study showed that it is possible to expand the pores of ordered mesoporous silica using decane or TMB but that the largest expansion, in this case a pore size of 7.6 nm, comes when these two swelling agents are added at the same time to the micellar solution. Alcohols Alcohols like ethanol and methanol are biproducts of the synthesis when using TEOS or TMOS as silica precursors. A study made by Denkova et al [31] shows that the presence of ethanol give a more homogenous product and that without ethanol, spherical micelles are present. They showed that the length of the silica 2.4. PORE CONTROL 13 worms depends on the amount of ethanol in the solution and the maximum length is given at a ethanol concentration at 8-10%. During a regular synthesis the ethanol concentration after complete hydrolysis of the TEOS is 10%. The result from Denkova et al in this case is not surprising. As mentioned in section 2.2.3 the ethanol goes into the core of spherical micelles, expanding the core and inducing the transformation from spherical to cylindrical micelles. A study of the hydrolysis processes of TMOS, TEOS and TPOS, Tetrapropyl oxysilane, in alcoholic solvents was performed by Bernads et. al. [32]. It was showed that under identical conditions the hydrolysis rate was decreasing with increasing length of the carbon chains and also with increasing amount of water in the solution. The alcohols used in this study were methanol, ethanol, 1-propanol and 2-propanol. The hydrolysis rate for each silane was the highest in methanol and lowest in 2-propanol. For ethanol and 1-propanol the rates were approximately the same. This is explained to be due to differences in proton activity of the catalyst in the solvents i.e. it is easier for the H+ to form H2 O with e.g. methanol than with ethanol or isopropanol due to the stronger bonds of the -OH group in these molecules. The hydrolysis rate is increasing with the water ratio for all of the alcohol solvents. Liu et al [21] used a combination of TEOS and sodium silicate in combination with ethanol to obtain highly ordered SBA-15 materials. They did not use the ordinary synthesis route; instead ethanol was dissolved in a buffer solution with pH 4.4. Then sodium silicate was added and stirred for 10 minutes before adding the TEOS. Thereafter the route was as usual with stirring for 20 hours and hydrothermaly treated for 24 hours. The pores in this material increased significantly. By changing the ethanol/P123 ratio from 0 to 426 the pore size was increased from 11 to 18 nm. This was explained by the fact that the hydrolysis rate of TEOS is faster in the absence of ethanol and that the hydrolyzed species of TEOS are hydrophilic and therefore not entering the PO core to swell the micelles. The ethanol is also acting as a co-surfactant, helping the swelling of the micelles. It should though be noted that the pore size distribution for this sample is very broad and has no sharp peak compared to other samples in this study. 2.4.3 Additional/alternative silica precursors Lower costs are always of great interest when it comes to production of materials. This is one of the reasons for finding alternative silica precursors when making SBA-15. Sodium metasilicate, Na2 SiO3 ·9H2 O, is one of these alternatives that has been studied. 14 CHAPTER 2. MESOPOROUS SILICA, SBA-15 As mentioned above, Liu et al [21] used a combination of TEOS and sodium metasilicate in a buffer solution with ethanol. They studied the effect of changing the ratio of TEOS/sodium silicate. One of the results from this study is that changing the TEOS/sodium silicate ratio one can tune the pore size in the range 10 nm for pure sodium silicate to 16 nm for pure TEOS. Fulvio et al [33, 34] have made two studies with pure sodium methasilicate materials have been produced. In these studies both samples made with TEOS and with sodium methasilicate were produced with the same conditions. The final products were similar in pore size but the sample made with sodium methasilicate had thicker pore walls and a lower pore volume. The thick pore walls are of great interest when it comes to thermal stability of the material. Also Kim and Stucky [35] used sodium silicate to make a range of SBA materials. Even this study showed that sodium silicate is a good alternative to TEOS or TMOS. 2.4.4 Salts Inorganic salts can be used both for controlling the morphology of SBA-15 [36] and for lowering the reaction temperature [37]. Yu et al [37] used KCl to synthesise ordered SBA-15 at a reaction temperature of 15◦ C. The resulting product had a smaller cell parameter and pore size than samples produced with the same molar composition but without KCl. They showed also that an increase in salt content can give an ordered structure at temperatures as low as 10◦ C. This is due to that the salt reduces the CMC of the polymers, making it possible for the surfactant to form micelles even in amounts below the CMC. What happens is that when ions are added to the solution, the EO chains of the polymer are more difficult to dissolve and thereby the CMC is decreasing [38]. The effect of the salts is therefore, as mentioned earlier, the same as from an increase in temperature. What really happens is that inorganic salts interact with the water molecules in the solution. If these interactions are strong, less water will be available to hydrate the surfactants and therefore the solubility of the surfactants is reduced [31]. The amount of interaction between the salt and the water depends on the type of anions and cations in the salt. A study has been made by Denkova et al [31] whith KCl, KF and KI. It was shown 2.4. PORE CONTROL 15 that KF had the strongest effect and that addition of KI did not lead to wormlike micelles. Their study also showed that the cations gave a smaller effect then the anions. The examined cations were Li+ , Cs+ and K+ where Li+ gave the smallest effect on the micelles. Many types of salts, such as NaCl, Na2 SO4 and K2 SO4 have been used to synthesize SBA-15 at low temperatures. A study of how fluoride affects the structure, both of the pores and the morphology of the particles, have been performed by Schmidt-Winkel et al [39]. They used NH4 F as a fluoride source and a F:Si molar ratio of 0.008 and 0.03. It was shown that a small amount of fluoride in the synthesis makes the silica to form large mesoporous rods if the pH is in the range of 0.4-1.2. These rods are made up by small, straight silica fibres. If the pH instead is in the range of 1.2-2.7, the silica fibres are bent and looks more like the fibres made in synthesis without the addition of fluoride. Schmidt-Winkel et al explains the change in morphology with a metastable nonequilibrium structure. They assume that the linear chain growth of the silica is promoted by the presence of small amounts of fluoride. When the F:Si ratio is 0.123 or higher, no ordered silica is formed. 2.4.5 Reaction conditions All studies show that the reaction conditions are crucial for the quality of the product. Parameters such as reaction time and temperature as well as pH and additives affect the delicate balance between the rate of hydrolysis of the silica precursor and the micelle formation. Reaction time As mentioned earlier, studies have been made of the formation process of the structure [17, 18, 19]. In these studies it was shown that the formation of the hexagonal structure and the silica walls are formed during the first two hours after adding the TEOS to the mixture. Two studies have been made by Fulvio et al [33, 34] where the reaction time has been decreased from 20 to 2 hours. It was shown that 2 hours is enough for the self-assembly and the result was high quality SBA-15 with a pore size of 10.5 nm. pH There have been attempts to form ordered mesoporous materials over the whole pH-range [6]. In an acid media the mesoporous silica is formed but when the pH is increased to the range 2-6, no precipitation of silica is observed. If the solution is 16 CHAPTER 2. MESOPOROUS SILICA, SBA-15 neutral, disordered mesoporous silica is formed after calcination and if the media is basic, only amorphous silica or silica gels are formed. It has also been shown for pH<1 HCl, HI, HNO3 , S2 SO4 and H3 PO4 all give a ordered silica structure [7]. Reaction temperature Kipkemboi et al [26] showed that the hydrophobicity of the polymer is strongly affected by the temperature and therefore the temperature influences the particle size. As the temperature is raised, the EO chains become less soluble in water and hence the molecule becomes more hydrophobic. A higher synthesis temperature will affect the pore shape but not the size. The higher temperature the lower curvature of the structure will be, i.e. a higher packing number. If the temperature is close to the cloud temperature, i.e. the temperature limit for formation of ordered material, the material formed will be of bad quality and largely unaffected by the templating agent. But the temperature does not only affect the pore structure, the unit cell of the hexagonal silica structure is also increased with increasing synthesis temperature. Brodie-Linder et al [40] made a study of how lasting effects of temperature change within the first 10 minutes of the TEOS polymerization. They showed that a higher temperature during the first 10 minutes results in a lower microporosity in the final product while a lower temperature than the following reaction temperature do not give any pronounced effect on the product. This was explained by that the polymerization rate of TEOS increases with increasing temperature. A change from 35◦ C to just 55◦ during the first 10 minutes of the reaction makes a denser silica wall. This makes it more difficult for the EO chains to make impressions in the wall and thereby gives a lower microporosity in the final product. 2.4.6 Hydrothermal treatment Several groups report that an increase in hydrothermal time or temperature increases slightly the pore diameter and reduces the silica wall in SBA-15. This phenomena was noticed in the first article about SBA-15 [7] and several studies of this mechanism have been performed. The process reduces the shrinkage of the pores during calcination [14, 29]. If this step is removed during the synthesis, the nitrogen adsorption will show a broad hysteresis loop which indicates constrictions in the mesopores [29]. The same result appears if there is an excess of silica source during the synthesis [41]. But the hydrothermal treatment affects not only the pore size, also the unit cell parameter and the microporosity of the product is affected by changes. A study with SBA-15 synthesized in the presence of hexane was performed by 2.4. PORE CONTROL 17 Kruk and Liang [29]. They studied the effect of hydrothermal treatment time and temperature from 3 hours to 5 days and 40◦ C to 130◦ C and compared these results with samples without any treatment. The study showed that to change the shape of the broad hysteresis loop, any form of hydrothermal treatment is needed. When the treatment exceeds 3 hours at 100◦ C or 40◦ C for 1 day the loop becomes more narrow and shows cylindrical pore with a small pore size distribution. Pore size Fulvio et al [34] made a study where both hydrothermal temperature and time were changed. The study was performed using SAXS and nitrogen adsorption. They showed that an increase of hydrothermal treatment time, from 6 to 120 hours, increases the pore width, at first rapidly but after 48 hours the increase was flattened. At the same time there is a linear decrease of both BET surface area and wall thickness of the products. A higher hydrothermal treatment temperature, 120◦ C, gave a slightly higher mesopore volume and larger mesopore width but the increase in pore width with increasing time was not as pronounced as for 100◦ C samples. Galarneau et al [42] have studied the effects of hydrothermal treatment temperature with a temperature span from 35 to 140◦ C. The pore size was determined by the Broekhoff and de Boer method [43]. They showed that samples treated for 48 hours at low temperatures, 35-60◦ C, have the same pore size, but above 60◦ C the sizes increases linearly with temperature. It therefore seems like the largest pore size would be gained with a high hydrothermal treatment temperature. The time for the hydrothermal treatment does not seem to be as important as long as the temperature is high enough. Unit cell parameter Also the unit cell parameter was studied by Fulvio et al [34]. They showed that all samples shrink upon calcination, but the amount of shrinkage depends on the hydrothermal treatment. An untreated sample has a unit cell parameter that is 15% smaller than the uncalcinated sample. This can be compared with a shrinkage of 5% for a sample that is treated 1 day in 100◦ C or 2-3% for a sample treated for 5 days at 130◦ C. Similar result was given by Galarneau et al [42]. They noted though that treatment temperatures below 60◦ C gives a shrinkage of 20% and 5% for samples treated above this temperature. The most interesting thing from this study is that the cell parameter is approximately constant for all samples treated above 60◦ C, which means that the walls in the material shrink at the same rate as the pore ex- 18 CHAPTER 2. MESOPOROUS SILICA, SBA-15 pands. For samples treated below 60◦ C the unit cell parameter is also constant, but this is also the case for the pore width. Microporosity It is clear that with a longer hydrothermal treatment time or higher temperature, the microporosity of SBA-15 decreases. This has been shown by for example Galarneau et al [42]. When the unit cell parameter is constant and the pores increases, the silica walls gets thinner. The model of Vradman et al [16] says that this still decrease the microporosity, so that is one possible explanation of the decrease in microporosity. Another explanation of this phenomenon is the coronal model where the micropores are due to EO chains trapped in the silica walls. In this model the EO chains goes into the centre of the micelle during the hydrothermal treatment. Then, during calcination, the voids from the EO chains in the silica walls are partly filled by silica during the shrinking of the unit cell, and thereby reducing the microporosity. Mechanism The reason for changes in pore size and microporosity seems to be quite well understood. During the hydrothermal treatment the expansion of the organic core is realized by pushing out of the organic layer in the silica walls [29, 34, 40]. The reason for this is that the hydrophobicity of the EO chains increases with increasing temperature. This leads to that the chains goes into the core of the micelles and thereby expands them, pushing out the silica walls. During the calcination the heat treated samples, as mentioned earlier, have a lower shrinkage than untreated samples and this together with the expanded core give a larger pore size. So, during the hydrothermal treatment the EO chains in the synthesis with a higher initial reaction temperature will expand the core but not leave as many impressions in the walls compared to the synthesis with the lower initial reaction temperature. This results in a material with smoother pore walls with no microporosity. There are though still micropores bridging between the mesopores. These micropores are due to trapped EO chains in the silica walls and are not made by impressions during the hydrothermal treatment. 2.5 Summary SBA-15 is one type of mesoporous silica with a hexagonal pore structure. The pores have a diameter in the range of 6.5-15 nm. In the silica walls there is a net- 2.5. SUMMARY 19 work of micropores that connects the mesopores with each other. The micropores are assumed to be due to EO chains from the surfactant that have been trapped inside the walls during the formation. The structure of SBA-15 is formed by micelles made by an amphiphilic block copolymer, P123, in an acidic solution containing water and HCl. A silica precursor, TEOS, is added to the solution and starts to build a layer of silica around the spherical micelles. The micelles become elongated and are packed in a hexagonal structure. During the calcination the P123 is burned off and porous silica is formed. It is shown that there are many different ways to affect the final product of SBA-15. All four steps: synthesis, hydrothermal treatment, drying and washing and finally the calcination are crucial for the properties of the final product. During the synthesis, the structure of the material is formed. The formation of micelles is affected of many parameters such as reaction temperature and pH. Also additions of inorganic salts and swelling agents affect the synthesis by changing the critical micelle concentration and expanding the micelles. The silica precursor is affected by addition of alcohols, pH and reaction temperature. The hydrothermal treatment is used for stabilizing the structure. Without this step the material shows large hysteresis loops. By changing the time or temperature in this process one can tune pore size and wall thickness in the material. This has been shown in several studies and seems to one of the best ways of reliable expansion of the pore diameter. Another way of expanding the pore size is to use swelling agents such as TMB, ethanol or alkenes. TMB has been used to expand the pores to 12 nm but at higher amounts of TMB, there is a phase transition in the material and a mesocellular foam is formed. Alkenes have been used as swelling agents at low reaction temperatures and SBA-15 has been formed with a pore size of 15 nm. 20 CHAPTER 2. MESOPOROUS SILICA, SBA-15 Chapter 3 Experimental design 3.1 Synthesis In a typical synthesis, 50.07 g distilled water, 35.31 g 4 M hydrochloride acid and, if any, X ml swelling agent were mixed in a flask and heated to 40◦ C during stirring. When the mixture reached 40◦ C 2.5 g P123 was added and the stirring continued until the polymer was dissolved. Then 4.1 ml TEOS was added to the solvent during vigorous stirring, approximately 1000 rpm. After stirring at the same temperature for 20 hours the temperature was raised to 100◦ C, the flask was equipped with a condenser and the stirring was turned off. After 24 hours, the product was filtered, washed with distilled water and dried in air. The time for drying was approximately 4–5 days. When the product had dried it was calcinated in 560◦ C for 5 hours. The ramp was 10◦ C/min and after calcination the furnace cooled down to room temperature before the product was removed. Before any characterisation of the product it was crushed in a mortar. 3.1.1 Samples The produced samples and their synthesis conditions are seen in table 3.1. 21 CHAPTER 3. EXPERIMENTAL DESIGN 22 Table 3.1: All samples produced and their synthesis conditions. Sample Additive ME24 ME72 ME24-3M ME48-3M ME72-3M ME24-3M-PH1 ME24-3M-PH2 ME24-3M-125A ME24-3M-140A ME24-6M ME24-3E ME24-3E-35R ME24-3E-45R ME24-3E-50R ME72-3E ME72-3E-45R-125A ME72-3E-45R-140A ME24-6E ME24-6E-35R ME24-6E-45R ME24-3I ME72-3I ME24-6I ME24-3F ME24-3A ME24-6A ME24-3T Methanol Methanol Methanol Methanol Methanol Methanol Methanol Methanol Ethanol Ethanol Ethanol Ethanol Ethanol Ethanol Ethanol Ethanol Ethanol Ethanol Isopropanol Isopropanol Isopropanol Formaldehyde Acetone Acetone THF Additive:P123 ratio 0 0 210 210 210 210 210 210 210 420 210 210 210 210 210 210 210 420 210 210 210 210 420 210 210 420 210 Reaction temperature [◦ C] 40 40 40 40 40 40 40 40 40 40 40 35 45 50 40 45 40 40 35 45 40 40 40 40 40 40 40 Ageing time [h] 24 72 24 48 72 24 24 24 24 24 24 24 24 24 72 72 72 24 24 24 24 72 24 24 24 24 24 3.1.2 Additives The additives used as swelling agents in this study are methanol, ethanol, isopropanol, formaldehyde, acetone and tetrahydrofuran, THF. The structures of these molecules are seen in figure 3.1. Some properties of these elements are seen in table 3.2. Ageing temperature [◦ C] 100 100 100 100 100 100 100 125 140 100 100 100 100 100 100 125 140 100 100 100 100 100 100 100 100 100 100 3.2. X-RAY DIFFRACTION 23 (a) Methanol (b) Ethanol (c) Isopropanol (d) Formaldehyde (e) Acetone (f) THF Figure 3.1: Structure of the swelling agents used (a) methanol, (b) ethanol, (c) isopropanol, (d) formaldehyde, (e) acetone and (f) THF. Table 3.2: Properties of swelling agents. Relative Melting Boiling Solubility density point point in water Methanol 0.79 -98 65 Miscible Ethanol 0.80 -117 79 Miscible Isopropanol 0.79 -90 83 Miscible Formaldehyde -117 -19 Very good Acetone 0.80 -95 56 Miscible THF 1.05 -80 178 Good Reagent Dipole moment 1.69 1.69 1.66 2.33 2.91 1.63 3.2 X-Ray Diffraction Measurements with X-Ray Diffraction (XRD) show if there are a crystalline structure of the pores in the mesoporous material. The basic principle of XRD is the Bragg condition nλ = 2dhkl sin θ. λ is the wave length of the X-rays. (3.1) 24 CHAPTER 3. EXPERIMENTAL DESIGN dhkl is the distance between the planes with indexes h, k and l. θ is the angle at which constructive interference occurs. The sample is scanned in a range of θ and the intensity of the outgoing beam is detected. This gives a spectrum with peaks for constructive interference. In this case XRD is used to receive information about the d spacing. Since SBA-15 has a hexagonal symmetry lattice the (100), (110) and (200) usually can be seen in the range of 0◦ -3◦ [21, 44, 45]. An increase of the d(100) spacing indicates an expansion of the hexagonal unit cell [21]. In this study a Phillips diffractometer was used with an X-ray source that was a Cu target tube with Ni filters which gives the Kα wave length of Cu, λ=1.54 Å. The voltage was 40 kV and the current 40 mA. The scan range used was 0.74◦ 2.5◦ , with a step size of 0.020◦ and a time per step of 25 s. 3.3 Physisorption In this study a Micrometrics ASAP 2020 was used for the physisorption measurements. In physisorption a gas is adsorbed on the surface of the sample through Van der Waals interactions. Analysing the amount of adsorbed gas at different pressures, isotherms, gives information about the surface area and pore structure. The sample is first degassed to remove all moist. This was done in the ASAP 2020 at 300◦ C for 4 hours with a temperature ramp at 10◦ C/min. Then the free volume of the sample tube is measured by introducing helium gas in the tube. Both the warm free space, at room temperature, and cold free space, when the sample is cooled with liquid nitrogen, are measured. After measuring the free space all helium gas is removed from the sample tube and nitrogen gas is added to the sample in a series of controlled pressures. First, the micropores are filled with gas molecules that condenses on the surface and then the free surfaces are covered with condensed nitrogen. When enough amount of nitrogen is added also the large pores are filled. This process continues until the P/P0 is close to 1. At this pressure the total pore volume is measured. Now, the desorption starts. Also this process is carried out in a series of controlled pressures. The pressure is reduced and the adsorbed molecules are released. During both the adsorption and desorption process, the amount of adsorbed gas is determined by using pressure at which adsorption equilibrium occurs and applying the universal gas law. This result in one adsorption and one desorption curve called isotherms. The isotherms give information about the surface characteristics 3.3. PHYSISORPTION 25 Figure 3.2: Types of physisorption isotherms [3]. and porosity of the material. 3.3.1 Adsorption isotherms Most physisorption isotherms can be grouped into six categories which are seen in figure 3.2. The fourth category is associated with mesoporous materials. The characteristics of this isotherm are the hysteresis loop and the limiting uptake at high pressures [3]. The type IV isotherm can be divided into three parts. At first, the isotherm increases rapidly when a monolayer of gas covers the material. The second part is almost linear and is due to multilayer adsorption. The hysteresis loop is the third part, which is associated with capillary condensation in the mesopores. There are different shapes of the hysteresis curves, which can be seen in figure 3.3, associated with specific pore structures [3]. The distance between adsorption and desorption in this loop gives information about the pore size distribution [29] and plugs in the pores [41]. 3.3.2 BET Surface Area The BET specific surface area [46] is calculated using adsorption isotherms in the relative pressure range 0.05 to 0.25. The BET area is expressed by the BET CHAPTER 3. EXPERIMENTAL DESIGN 26 Figure 3.3: Types of hysteresis loops [3]. equation 1 C −1 p/p0 = + (p/p0 ). n(1 − p/p0 ) nm C nm (3.2) p/p0 is the relative pressure, n is the amount adsorbed, nm is the maximum amount adsorbed and C is a constant. The plot of the left expression in the equation against p/p0 gives a straight line with the slope s= C −1 . nm C (3.3) The BET surface area is then calculated from the relation BET = nm Na σ (3.4) where Na is Avogadro’s number and σ is the average area occupied by one molecule. 3.3.3 BJH Pore Volume The method developed by Barrett, Joyner and Halenda, BJH method, is used for calculating the pore size and diameter. In this method each pore filled with condensed liquid nitrogen is considered to have three zones which can be seen in figure 3.4. The core is the volume which evaporates all at once when the critical pressure for that radius is reached. The adsorbed layer is composed of adsorbed gas that is stripped off a bit at a time with each pressure step. The wall of the cylindrical pore is the diameter of the empty pore which is required to determine the pore volume 3.3. PHYSISORPTION 27 Figure 3.4: A pore filled with condensed liquid nitrogen has three zones: the core, the adsorbed layer and the walls of the cylindrical pores. and area. The end area of each pore is neglected since it is much smaller then the length of the pore. Total pore volume is estimated at the relative pressure of 0.95 3.3.4 KJS pore size analysis The Kruk-Jaroniec-Sayari, KJS, method for calculating the pore size distribution from gas adsorption isotherms assuming cylindrical pore size geometry [47]. This method has been successfully for MCM-41 materials which have a smaller pore size than SBA-15 and MCM-41 also lacks the interconnecting microporous network found in SBA-15. This method can be applied also for SBA-15 materials but gave an overestimation of the pore size for this material. The method was further developed and in 2006 one found a improved model that gives a distribution even for SBA-15 materials that fits results from SAXS and XRD measurements [48]. In the KJS method one evaluate the pore width w by using the relation between the unit cell parameter a and the volume of ordered mesopores evaluated on the nitrogen adsorption isotherm. In the software in ASAP 2020 the original KJS method is used and therefore all results reported will be calculated by this method. In this case the pore width is estimated by using x+y =z (3.5) w = −a/[log (bp/p0 )] + 2t + c (3.6) where a, b and c are parameters best fitted [48] and t is the statistical thin film CHAPTER 3. EXPERIMENTAL DESIGN 28 thickness of nitrogen adsorbate t(p/p0 ) = wd 1− 2 à !1/2 vp, max − vp (p/p0 ) . vp, max (3.7) wd is the size of primary mesopores and is calculated with à ρVp wd = cd 1 + ρVp ! (3.8) where d is the (100) spacing obtained from XRD, ρ is the density of the walls and c = (8/(31/2 π))1/2 . This equation was derived for the infinite structure of uniform cylindrical pores with the diameter wd arranged in a hexagonal pattern with the (100) interplanar spacing d [47]. vp (p/p0 ) is the amount adsorbed in primary mesopores as a function of the pressure vp (p/p0 ) = v(p/p0 ) − Sex SBET, ref vref (p/p0 ) (3.9) where SBET, ref and vref (p/p0 ) are the BET specific surface area and the adsorption isotherm for the reference adsorbent used. vp, max is the maximum adsorption capacity of primary mesopores. This leads to a film thickness curve expressed as · t(p/p0 ) = 0.1 60.65 0.03071 − log p/p0 ¸0.3968 (3.10) and the pore size distribution is calculated by r(p/p0 ) = 2γVL + t(p/p0 ) + 0.3 nm. RT ln (p/p0 ) (3.11) 3.4 Scanning electron microscopy With a Scanning Electron Microscope, SEM, it is possible to study the structure of the material. It is not possible to see the pores due to lack of resolution, but it is possible to study the grain and the shape of these. In an SEM a beam of electrons produced by an electron gun goes through two condenser lenses, a scan coil and an objective lens to finally interact with the samples. In the sample the electrons lose energy due to scattering and absorption in the sample. The depth of electron penetration into the sample depends on the landing energy of the electrons and the material of the sample. If the sample is a semi 3.5. TRANSMISSION ELECTRON MICROSCOPY 29 conductor or insulator the sample can be charged by the electron beam. This is the case of our samples and therefore they were coated with a thin layer of gold using sputtering. Each pixel in the resulting image shows the intensity of the electrons that are ejected back from the sample for each spot on which one measures. The intensity is given a greyscale value and when scanning over an area the image will be formed pixel by pixel. In this study a Leo 1550 Gemini Scanning Electron Microscope was used. The working distance was 3-4 mm and the electron voltage 3.0 kV. 3.5 Transmission electron microscopy Transmission electron microscope, TEM, is in this study used to see the pore structure of the samples. A FEI Tecnai G2 TF 20 UT 200 kV field emission gun microscope was used in this study. A TEM works similarly as an SEM, but instead of looking at ejected electrons from the surface, the electron beam goes through the sample. After passing the sample, the image formed by the electrons is magnified and focused by an objective lens and images on a fluorescent screen. To make it possible for the electron beam to go through the sample, the sample needs to be very thin. In this study the material is a powder and can therefore not be polished. Instead the powder was added to a small amount of acetone and put in an ultrasonic bath for 10-15 minutes. In the ultrasonic bath the lightly agglomerated particles are shaken apart and separate particles are obtained in the solution. The fluid are then dropped onto a TEM sample holder which is a carbon grid. The particles get stuck onto the grid and remains there without any other preparations. 3.6 Fourier transformed infrared spectroscopy Fourier transformed infrared spectroscopy, FT-IR, was used to see the functional groups in the products and to see if all P123 is burned off during the calcination. With FT-IR it is possible to study the vibrations and rotations of atoms in molecules due to their discrete energy levels. This energy corresponds to a frequency which are determined by the masses of the atoms and the type of movement. To make measurements with FT-IR the powdered product must be formed into a pellet. This is done by mixing 120 mg KBr, which is invisible for the radiation, with 0.7-1.0 mg sample. The product and KBr and mixed and crushed together 30 CHAPTER 3. EXPERIMENTAL DESIGN with a mortar. The powder is put in a holder and pressed together to form a pellet. The measurement was performed in the range of 400-4000 cm−1 with a step size of 2cm−1 . Chapter 4 Results All samples have been characterised with earlier mentioned techniques. For an overview of all results see appendix A.1. 4.1 Original sample The samples without any additives and made at ordinary temperatures, 40◦ C for reaction temperature and 100◦ C hydrothermal treatment temperatures. One sample was made with 24 hours hydrothermal treatment time and one with 72 hours. These samples are called ME24 and ME72, respectively. Figure 4.1 shows a characteristic microstructure of approximately 1 µm elongated particles, loosely agglomerated into big particles. Figure 4.1: Scanning electron micrograph of (a) ME24 and (b) ME72 Figure 4.2 shows the characteristic pore structure (a) along and (b) a cross section of the pores. It is clear that the samples have cylindrical pores arranged in a hexagonal pattern. 31 32 CHAPTER 4. RESULTS Figure 4.2: Transmission electron micrograph of (a) ME24 and (b) ME72. In figure 4.3, the nitrogen adsorption-desorption isotherms are shown for ME24 and ME72. Both samples show a pure type IV adsorption isotherm curve with a type 1 hysteresis loop which indicates pure cylindrical pores in both samples. Figure 4.3: Nitrogen isotherms of ME24 and ME72. Both samples have a similar d(100) spacing, 8.8 and 9.0 nm for ME24 and ME72 respectively. The pore size distribution in figure 4.13 shows that the pore size is approximately 6.4 nm for ME24 and 7.3 nm for ME72. This gives that the pore walls thicknesses are 3.8 and 3.2 nm for ME24 and ME72. 4.2. SWELLING AGENTS 4.2 33 Swelling agents Six different swelling agents, methanol, ethanol, isopropanol, formaldehyde, acetone and THF have been used in this study. A comparison was made between the materials with different additives where the molar ratio between additive and P123 was 210. This was followed by another comparison where the amount of additive, in this cases the alcohols and acetone, was doubled. 4.2.1 Different additives All samples with the lower ratio have an ordered pore structure which can be seen in figure 4.6. From these images it is clear that all samples contain not only the ordered structure but also some foam with unstructured pores. Figure 4.4: Nitrogen isotherms for samples with swelling agents. In figure 4.5 one can see that the amount of order differs between the additives. The sample with the largest pore size, 8.3 nm, is ME24-3F while ME24-3A is the sample with the largest amount of pores at its maximum pore size of 7.8 nm which indicates that this is the most ordered sample. This is confirmed by the best hysteresis loop in figure 4.4. 34 CHAPTER 4. RESULTS Figure 4.5: Pore size distributions for samples with swelling agents. The sample with the lowest order and also smallest pore size is ME24-3T with 7.7 nm in pore diameter. The low order in the sample is seen in form of a low and broad pore size distribution and also in the lack of step in the hysteresis loop. The wall thickness of all samples with additives are smaller than the 3.8 nm for ME24. ME24-3M has the thickest walls with 3.2 nm. For the rest of the samples the thicknesses are 1.3, 1.9, 2.5, 1.3 and 2.0 nm for ME24-3E, ME24-3I, ME243F, ME24-3A and ME24-3T respectively. The thicknesses are calculated from the d(100) spacing and that these results are unsure due to difficulties to get a clear peak in the X-ray diffraction. In figure 4.7 the microstructure of samples with additives is shown. It is clear in figure 4.7(a)-(e) that the samples contains of elongated particles that are loosely agglomerated and in all cases covered with foam. The particles are approximately 1µm long and 100-200 nm in diameter. In figure 4.7 (f) the microstructure of ME24-3T is seen. It is clear that this sample presents another morphology than the other samples, with particles that look more chubby with a size of approximately 0.5 µm. Also in this case the particles are covered with foam. The hysteresis loop of ME24-3T contains no sharp step and the morphology of the sample differs from all other samples. The TEM image 4.6(f) shows that there are some ordered pores but the majority of the sample is unordered which is confirmed by the pore size distribution. 4.2. SWELLING AGENTS 35 Figure 4.6: Transmission electron micrograph of (a) ME24-3M (b) ME24-3E (c) ME24-3I (d) ME24-3F (e) ME24-3A and (f) ME24-3T. 36 CHAPTER 4. RESULTS Figure 4.7: Scanning electron micrograph of (a) ME24-3M, (b) ME24-3E, (c) ME24-3I, (d) ME24-3F, (e) ME24-3A and (f) ME24-3T. 4.2. SWELLING AGENTS 4.2.2 37 Increasing the amount of additives When the amount of additives is doubled the pore sizes are not increasing. The pore size distributions in figure 4.9 show that the maximums are at 8.2 nm for ME246E, ME24-6I and ME24-6A while for ME24-3M the maximum is at 7.3 nm. The d(100) spacings for these samples are approximately 82±1 nm. This gives a wall thickness of approximately 1.5 nm for ME24-6E, ME24-6I and ME24-6A and for ME24-6M, 2.3 nm. Figure 4.8: Nitrogen isotherms for ME24-6M, ME24-6E, ME24-6I and ME24-6A. Figure 4.9: Pore size distributions for ME24-6M, ME24-6E, ME24-6I and ME246A. From the nitrogen isotherms in figure 4.8 it is clear that the order in the samples is decreasing when the amount of additives is increased. None of the hysteresis loops 38 CHAPTER 4. RESULTS looks like pure type 1. Instead, all samples show combinations of type 1 and type 4 hysteresis loops. In figure 4.10, transmission electron micrographs are showed. They reveal that there is some ordered porosity in all samples except for ME24-6M which mostly consists of foam. The microstructure of ME24-6M and ME24-A are seen in figure 4.11. A comparison of this image and figure 4.7 (a) and (e) shows that the amount of foam is increased when the amount of additive is increased. Figure 4.10: Transmission electron micrograph of (a) ME24-6M, (b) ME24-3E, (c) ME24-6I and (d) ME24-6A. 4.2. SWELLING AGENTS 39 Figure 4.11: Scanning electron micrograph of (a) ME24-6M and (b) ME24-6A. 4.2.3 Hydrothermal treatment of samples with additives Time Increasing the hydrothermal treatment time is from literature known to increase the pore size and reduce the wall thickness of SBA-15. Figure 4.12 shows that the order in the samples is increased when the hydrothermal treatment time is increased form 24 to 72 h. The pore sizes for ME24-3M, ME72-3M, ME24-3E, ME72-3E and ME72-3I are approximately the same, 8.2 nm, which can be seen in figure 4.13. This is as mentioned above slightly larger than for ME24-3I. The d(100) spacing varies more than the pore size. The largest spacing is for ME24-3M, 9.9 nm, followed by 9.6 nm for ME72-3E. For ME72-3I it was not possible to measure the d(100) spacing. The rest of the samples have d(100) spacing of approximately the same size, 8.2±1 nm for ME72-3M, ME24-3E and ME24-3I respectively. The wall thickness for ME24-3M and ME72-3E are thicker, 3.2 and 2.9 nm respectively, than for the other samples that have a wall thickness of 1.2-1.9 nm. This results are much lower than for ME24 and ME72 that have been produced at the same conditions that these samples but without any additives. 40 CHAPTER 4. RESULTS Figure 4.12: Nitrogen isotherms for (a) ME24-3M and ME73-3M, (b) ME24-3E and ME72-3E and (c) ME24-3I and ME72-3I. 4.2. SWELLING AGENTS 41 Figure 4.13: Pore size distributions for (a) ME24 and ME72, (b) ME24-3M and ME73-3M, (c) ME24-3E and ME72-3E and (d) ME24-3I and ME72-3I. 42 CHAPTER 4. RESULTS Temperature When the hydrothermal treatment temperature is increased from 100 to 125◦ C, the order in the samples is increasing when the hydrothermal treatment time is 24 h. This can be seen in the nitrogen isotherms in figure 4.14(a). When instead the treatment time is 72 h, there are not such a pronounced difference between hysteresis loops of the samples, see figure 4.14(b). Figure 4.14: Nitrogen isotherms for (a) ME24-3M, ME24-3M-125A and ME243M-140A and (b) ME72-3E, ME72-3E-45R-140A and ME72-3E-45R-140A. Increasing the hydrothermal treatment temperature from 100◦ C to 125 or 140◦ C increases the pore sizes for samples with methanol and ethanol as swelling agents. This can be seen in figure 4.15. Unfortunately there are too few measuring points at higher pore widths to see clearly what the maximums are and how much the pores are increasing, but it is still clear that they are. 4.2. SWELLING AGENTS 43 Figure 4.15: Pore size distributions for (a) ME24-3M, ME24-3M-125A and ME243M-140A and (b) ME72-3E, ME72-3E-45R-140A and ME72-3E-45R-140A. The d(100) spacing for the methanol samples are decreasing from 9.9 nm to 8.7 and 8.5 nm for ME24-3M-125A and ME24-3E-140A respectively. The same trend is seen in the samples with ethanol. Here the d(100) spacing decreases from 9.6 to 9.0 and 8.3 nm for ME24-3E-45R-125A and ME34-3E-45R-140A respectively. This decrease in d(100) spacing with increasing hydrothermal treatment temperature combined with an increasing pore size gives a significant decrease in wall thickness. 44 CHAPTER 4. RESULTS 4.3 Reaction temperature The reaction temperature is an important parameter. This can be seen in the study made with ethanol as a swelling agent and a reaction temperature in the range 3550◦ C. The effect is very clear in the SEM images, see figure 4.18 and 4.19. In this study both the low and high amount of ethanol have been used in the samples. The pore size distribution of these samples, figure 4.17, shows that the size of the pores does not change with increasing temperature but the distribution becomes narrower. In this case it looks like if a high reaction temperature gives a cleaner sample. Figure 4.16: Isotherms for samples with (a) the lower amount of ethanol produced with 35, 40, 45 and 50◦ C and (b) the higher amount of ethanol produced at 35 and 45◦ C reaction temperature. 4.3. REACTION TEMPERATURE 45 Figure 4.17: Pore size distributions for samples with (a) the higher amount of ethanol produced at 35 and 45◦ C and (b) the higher amount of ethanol produced at 35 and 45◦ C reaction temperature. 46 CHAPTER 4. RESULTS Figure 4.18: Scanning electron micrograph of (a) ME24-3E-35R, (b) ME24-3E, (c) ME24-3E-45R and (d) ME24-3E-50R. Figure 4.19: Scanning electron micrograph of (a) ME24-6E-35R and (b) ME246E-45R. 4.3. REACTION TEMPERATURE 47 Figure 4.20: Transmission electron micrograph of (a) ME24-3E-35R, (b) ME243E, (c) ME24-3E-45R and (d) ME24-3E-50R. Figure 4.21: Transmission electron micrograph of (a) ME24-3E-35R, (b) ME243E, (c) ME24-3E-45R and (d) ME24-3E-50R. CHAPTER 4. RESULTS 48 4.4 pH The pH in the reaction was modified by changing the amount of HCl. The isotherms in figure 4.22 and pore size distributions in figure 4.23 shows that there are almost no order in these samples. There is a small peak in the pore size distribution for ME24-3M-PH1 with a maximum at 7.8 nm, but it is very low. The hysteresis loops for both samples look not like a type hysteresis loop, but more like a type 2. The XRD does not show any peak which indicates that there is no ordered porosity in the material. This is confirmed by the TEM images in figure 4.24. In these images it is clear that there are pores in ME24-3M-PH1 even though they are not cylinders that follow the particles and that ME24-3M-PH2 only consists of foam. In figure 4.25 the microstructure of the samples synthesised with different pH is showed. Figure 4.22: Nitrogen isotherms for ME24-3M-PH1 and ME24-3M-PH2. Figure 4.23: Pore size distributions for ME24-3M-PH1 and ME24-3M-PH2. 4.4. PH 49 Figure 4.24: Transmission electron micrograph of ME24-3M-PH1 and ME24-3MPH2. Figure 4.25: Scanning electron micrograph of ME24-3M-PH1 and ME24-3MPH2. 50 CHAPTER 4. RESULTS 4.5 Calcination A comparison between the calcinated and uncalcinated samples is seen in figure 4.26. Figure 4.26: A comparison of the infrared transmission spectra for calcinated and unclalcinated samples. For both calcinated and uncalcinated a broad band around 3350 cm−1 appears. This band is in the calcinated samples completely due to the stretching mode of O-H in water molecules. In the uncalcinated samples it is partially due to this vibration but also to C-H stretching in the P123. The bending vibration of the O-H in water molecules is seen as a band at 1630 cm−1 . Silica gives a band at 468 cm−1 due to the Si-O-Si rocking mode, at 808 cm−1 is the O-Si-O bending mode and at 1000-1300 cm−1 the band is due to asymmetric stretching in Si-O [49]. Around 960 cm−1 there is a band that is more intense for the uncalcinated sample. This is due to the Si-OH bending which indicates that calcination decreases the number of silanol groups on the pore wall surfaces [50]. The bands that are not present in the calcinated spectrum are due to vibrations in the P123. The band around 2850-3000 cm−1 depends on the C-H stretching and at 1350-1500 cm−1 bending vibrations of the same bond. Chapter 5 Discussion 5.1 Original sample The samples made with a standard route and the resulting product show an ordered hexagonal pore structure. The pore size of the samples without addition of swelling agents are 6.4 nm for ME24 and 7.3 nm for ME72. The wall thickness of these samples are 3.8 and 3.2 nm for ME24 and ME72 respectively. The effect of hydrothermal treatment is an increase in pore size and a reduction in pore wall thickness. This is expected based earlier reported by e.g. Zhao et al, Clerc et al and Kruk et al.[7, 14, 29]. The general understanding of the effect of hydrothermal time is that longer exposure generates an increase in pore size. This is due to that the hydrophilic EO chains become less hydrophilic when the temperature is increased. When this happens, the EO chains starts to go into the core of the micelle and thereby expands it. This process takes a considerable amount of time which make it possible to expand the pore by increasing the hydrothermal treatment time for as long as 120 h [29, 34]. 5.2 Swelling agents As mentioned earlier, swelling agents have been used to increase the pore size of mesoporous silica. In this study, the effects of methanol, ethanol, isopropanol, formaldehyde, acetone and THF in the synthesis have been investigated. In this section, the samples with an additive/P123 ratio of 210 will be discussed followed by a discussion for samples with the doubled amount of additives in section 5.2.2 5.2.1 Different additives The hysteresis loops in the physisorption isotherms, figure 4.4 reveals that these products are quite poor in the ordered structure, even though the TEM images, 51 52 CHAPTER 5. DISCUSSION figure 4.6 shows ordered cylindrical pores to some extent. It is clear that the samples do not only contain cylindrical pores, but also a kind of foam with disordered, spherical pores. It should though be noted that the swelling agents not only affect the order in porosity and pore size, but also which reaction temperature and/or hydrothermal treatment that is optimal for the formation of large pore SBA-15. In a study of the formation process made by Ruthstein et al [20] it was shown that during the formation there is two phases coexisting in the solution. One phase is more silica rich and forms the cylindrical structure while the second phase is more diluted and consists of spherical micelles. There are though no, by the authors known, reports about foam in SBA-15 materials. When looking at the SEM images, figure 4.7 it is obvious that the particles are covered with foam. The extent of foam is depending on the swelling agent used. ME24-3A is the product with the least amount of foam, followed by ME24-3F. The samples containing alcohols have particles with ordered pores but they are all covered in foam. This sample shows type 1 hysteresis loop and also the volume in pore size distribution. The pore size distribution is though the widest of all samples, but it is a symmetric peak with a maximum at 8.4 nm which is an increase with 2 nm compared with ME24. SEM images show that this sample contains of discrete particles and the TEM reveals a highly ordered hexagonal structure. The second best additive in this study is formaldehyde. Also here, there is a type 1 hysteresis loop and a clear peak in the pore size distribution with a maximum at 8.0 nm. Samples with alcohols as swelling agents shows hysteresis loops that look similar to the type 1 hysteresis loops, but the loops are not coming together at a higher pressure as in a pure type 1. Instead it looks like a combination of a type 1 and a type 4 hysteresis loop. The type 4 is explained by slit like pores in the samples [3]. In this study the combination of hysteresis loops should be due to the combination of ordered cylinders and foam covering the particles. The pore distribution peaks for the methanol and ethanol samples have a maximum at 8.2 nm, but the intensity of the methanol peak is higher than for the ethanol. The isopropanol sample has a broad pore size distribution and no clear maximum, but the lack of a clear maximum can be due to too few measuring points. The maximum for this sample lies in the range of 7.3-8.0 nm. The step in the hysteresis loop is highest but still very small for methanol and more of a slope lower for ethanol 5.2. SWELLING AGENTS 53 and isopropanol. All the alcohol samples contain a lot of foam but show still an ordered structure in the TEM. Looking at the hysteresis loops in figure 4.4 and order the samples in order of likeness of type 1 loops gives the list: ME24-3A, ME24-3F, ME24-3M, ME24-3I, ME24-3E and finally ME24-3T. When looking in table 3.2 this follows the dipole moment of the swelling agents, the higher dipole moment, the more ordered sample. Water has a dipole moment of 1.8 D and hydrochloric acid 1.1 D. Our hypothesis is that when molecules such as acetone or formaldehyde which have higher dipole moment are added to the solvent the effect of ions will become higher. Both acetone and formaldehyde are soluble in water so they will not go into the core of the micelles. Instead they will be placed around the hydrophilic EO chains and repel them which will expand the size of the hydrophilic heads. When the hydrophilic heads are expanded the size of the micelles will expand and the additive works like a swelling agent. Alcohols on the other hand have a dipole moment below 1.8 D and will therefore not work in the same way as the acetone or formaldehyde. Our hypothesis is that the alcohol molecules are mixed with the water and when the P123 is added some of the alcohol molecules will come close to the hydrophilic EO chains, replacing water molecules. Since the methanol and ethanol are larger molecules than water they will expand the distance between the chains and thereby increase the size of the hydrophilic heads. But when isopropanol is used as a swelling agent, the size of it is so large that one isopropanol molecule needs to replace two water molecules. The dipole moment of the isopropanol is less than for water which will lead to a lower degree of swelling of the hydrophilic heads of the P123 and thereby not increase the pore size as much as methanol or ethanol when they are used as swelling agents. It is also clear that ME24-3M has a higher order than ME24-3E and ME24-3I. This effect can be explained by differences in the hydrolysis rate of the TEOS. It is known that alcohols decreases the hydrolysis rate of TEOS and that the rate increases with decreasing size of the alcohol molecule [32]. So, the reaction time for making SBA-15 is longer when alcohols are added. This can also explain the difference in wall thickness of the samples which is much higher, 3.2 nm, for ME243M and only 1.3 and 1.9 nm for ME24-3E and ME24-3I respectively. ME24-3M is the sample with the highest hydrolysis rate of the TEOS and has enough time in the synthesis to form mostly ordered pores in the sample. ME24-3E and ME24-3I contain a lot of foam which might indicate that the reaction is going too slow for a 54 CHAPTER 5. DISCUSSION complete ordered structure to form. Both these samples contain a high amount of foam which can be due to an incomplete formation of the hexagonal structure. Comparing ME24-3E and ME24-3I, it seems like ME24-3I have smaller pores and thicker walls than ME24-3E. The smaller pore size has already been explained above. The difference in wall thickness is very small and can be due to errors in the measurements, especially when finding the peak position in XRD. Ethanol has been used earlier as a swelling agent for SBA-15 [21]. In that study, a mix between TEOS and sodium silicate were used at silica precursors in a 4.4 pH buffer solution. The pore size when the only TEOS was used as silica precursor was 16 nm. The explanation given by Liu et al is that ethanol is acting as a swelling agent and also slows the hydrolysis of TEOS down so that the synthesis goes slower. This is not the result in our case which might be explained by the fact that we used an HCl solution with pH 0.5 and they used a buffer solution with pH 4.4. But, a higher pH decreases the hydrolysis rate of the TEOS and earlier studies [7] have showed that it is not possible to retrieve an ordered pore structure at pH>2. The reason for getting an ordered pore structure in the case of Liu et al could instead be due to something in their buffer solution that affects the formation of pores more than the hydrolysis rate of the TEOS. The final swelling agent used in this study is THF with a dipole moment of 1.3 D. This sample has a broad pore size distribution, with a maximum at 7.2 nm, and no clear step in the hysteresis loop. TEM images show that there are some ordered pores in the material but there is also a lot of foam. In the SEM image 4.7 it is clear that the product with THF does not look like materials with or without other swelling agents. There are no long particles; instead there are small lumps, sometimes covered with foam. It is clear from this study that, with these conditions, THF is not working as a swelling agent for SBA-15. 5.2.2 Increasing the amount of additives When the amount of acetone and alcohols were doubled, the structure of the sample is completely changed. The ordered structure is lost and only disordered foam is produced instead. This can be explained by that the dipole moment in the solution is too high which will lead to a so high repulsion of the hydrophilic head that they will not be able to form spherical micelles and thereby not form any ordered pores. 5.2. SWELLING AGENTS 5.2.3 55 Hydrothermal treatment Time An interesting observation was made when the hydrothermal treatment time was increased for samples with alcohols. The pore size is not increasing with hydrothermal treatment time as in the case without any swelling agents. This can be seen in the pore size distribution in figure 4.13 where all samples basically show the same pore size of 8.2 nm. It should though be noted that there can be small shifts in the pore size distribution that are not seen in the figure due to too few measuring points. In figure 4.13 it seems like the order in the samples is increasing with the hydrothermal treatment time. In all cases the amount of pores are increasing at the maximum compared with the samples treated a shorter time. A small increase in pore size from 7.4-8.0 nm to 8.2 nm are noted in the case with isopropanol as additive. This is though not happening in the cases of methanol or ethanol as swelling agents, in which the pore size seems constant. The fact that the order is increasing with time is confirmed by the hysteresis loops in figure 4.12. This figure shows the nitrogen isotherms for (a) ME24-3M and ME72-3M, (b) ME24-3E and ME72-3E and (c) ME24-3I and ME72-3I. In figure 4.12(a) it is clear that ME72-3M has a type 1 hysteresis loop in opposite to ME243M for which the hysteresis loop never closes at higher pressures. Figure 4.12(b) shows the same trend as for 4.12 but for the isopropanol samples in 4.12(c) the trend is not as clear. In this case, the step in the hysteresis loop is just slightly sharper for ME72-3I than for ME24-3I and it does not close at higher pressures as much as for ME24-3I. In figure 4.13(c) the pore size for these samples are of approximately the same broadness but shifted towards higher pore sizes for ME72-3I. The effect of prolonging the hydrothermal treatment time from 24 to 72 h for all samples with alcohols as swelling agents was studied. All previous studies, both with and without swelling agents, e.g. [7, 14, 29], shows that an increase in hydrothermal treatment time increases the pore size and decreases the microporosity in SBA-15. This study shows that this increase is not true for samples containing methanol or ethanol. Looking at the pore size distributions, figure 4.13 (a) and (b), it is clear that the only thing that happens when the time is prolonged is that the pore size distribution becomes narrower. It seems like the samples becomes more defined and well ordered which also is confirmed by the hysteresis loops in figure 4.12(a) and (b). For samples containing isopropanol, the pore size is slightly increased with increasing hydrothermal treatment time, see figure 4.13(c) but it seems like the order is lower with increasing time, see figure 4.12(c). 56 CHAPTER 5. DISCUSSION This is to our knowledge the first report of how an increase in hydrothermal treatment time does not induce an increase in pore size. Our hypothesis to explain this is that a solution including alcohol is not as water like as the ordinary solution without alcohols as additives. When the temperature is increased, the EO chains become less hydrophilic and in the ordinary synthesis the chains go into the micelle core, thereby expanding it. In the case with alcohols as swelling agents, the solution is enough non water like so that it does not repel the less hydrophilic EO chains. The result of this is that the EO chains do not go into the core of the micelles which thereby not will be expanded. To confirm this theory, a study of the microporosity of these samples should be made. The increased order in the samples when the hydrothermal treatment time is increased may be explained by the decrease in hydrolysis rate of the TEOS when alcohols are used as swelling agents. It might be that the reaction is not completed when the hydrothermal treatment is started and therefore it takes longer time in the hydrothermal treatment to form ordered structures. As can be seen in figure 4.16, the hysteresis loops are more like type 1 loops when the time is increased. In the study made by Kruk et al [29] the hysteresis loops are very broad before sufficient hydrothermal treatment time or temperature. The pore size distributions in their study clearly shows that the pore size distribution are mush lower for untreated samples than for samples made with sufficient hydrothermal treatment. Our hypothesis is further confirmed by the fact that the wall thickness for the ethanol samples are increased from 1.3 to 2.1 nm when the hydrothermal treatment time is increased. The time is obviously needed to for the walls and thereby increases the order in the samples. Temperature When it comes to hydrothermal treatment temperature, samples with methanol or ethanol have been treated at 100, 125 and 140◦ C. The pore size distributions for these samples, figure 4.15, contains too few measuring points to see sharp peaks at the higher temperatures but it is clear that in most cases a higher treatment temperature increases the pore size. For methanol, the sharpest peak in the pore size distribution comes from the sample treated at 125◦ C. At 140◦ C the pore size distribution is a lot broader but is also shifted to higher sizes with 1-2 nm, but it is not possible to see where the maximum is since there are too few measuring points. In the case of ethanol as swelling agent, the pore size distributions are shifted towards higher diameters both at 125 and 140◦ C. It seems like the maximum are approximately the same for both these temperatures, but it is not possible to say 5.2. SWELLING AGENTS 57 for certain since there are too few measuring points. It is though clear that the pore size distribution for these samples is much broader than for the sample treated at 100◦ C. In the study made by Kruk et al [29] it was shown that the pore size is increased when the hydrothermal treatment temperature is increased. They also showed that the effect of increasing temperature is less when higher hydrothermal treatment times are used. In our study, it is not possible to make any conclusions about this since there are too few measuring points in the pore size distribution which leads to unclear maxima in the graphs. It is though clear from the isotherms 4.14 that the order in the samples increases when the hydrothermal treatment temperature is increased from 100 to 125◦ C. This is especially clear in the samples treated for 24 h. When the hydrothermal treatment time is prolonged to 72 h the effect on order with increasing temperature is less pronounced. The reason for the increase in pore size when the temperature is increased is the same as for an increase in hydrothermal treatment time. The increase in temperature reduces the hydrophilicity of the EO chains which goes into the hydrophobic core of the micelle and expands it, thereby pushing out the silica walls. The samples produced with ethanol and high hydrothermal treatment temperature have a pore size in the range of 9.6-11 nm. It is not possible to be more exact since the amount of measuring points is too few. The d(100) spacing for these materials are 9.0 and 8.3 nm for ME24-3E-45R-125A and ME24-3E-45R-140A respectively. This gives a distance between neighbouring pores centres of 10.4 and 9.6 nm which is less then the diameter of one pore. This can be explained in two ways. The first is that the XRD-peaks are broad and not well resolved resulting in an inaccurate determination of the d(100) spacing. The other explanation is that we have used the original KJS method to calculate the pore size distributions. This method overestimates the pore size above 7 nm [REF]. A pore with the diameter 10 nm, measured with SAXS, will according to the original KJS method have a pore size of 11.3 nm which is an overestimation with 1.3 nm. This means that our pores should instead be in the range of approximately 8.89.8 nm if one make a rough estimation of the error of 9.6 and 11 nm. It is obvious that the pore walls in these samples are very thin. Kruk et al [29] also mentions that when the hydrothermal treatment temperature are increased, especially at 130◦ C, and a sample is made with micelle expanders, the micelles may be connected through large mesoporous gaps in the silica walls. This can lead to that 58 CHAPTER 5. DISCUSSION neighbouring mesopores can be seen as large voids and give a larger pore size than single mesopores. 5.3 Reaction temperature As mentioned earlier, alcohols such as ethanol decreases the hydrolysis rate of TEOS. Another parameter that affects the rate is the reaction temperature [40]. In our study the samples containing ethanol have been synthesized at 35, 40, 45 and 50◦ C reaction temperature. It is obvious from the physisorption data, figures 4.16 and 4.17, that something happens when the reaction temperature is increased to 50◦ C. The hysteresis loop for this sample is a very clear type 1 and there is a sharp peak in the pore size distribution. Looking at the other samples with ethanol, the hysteresis loops are very poor and the pore size distributions not as intense as for the 50◦ C sample. Looking at SEM images, figure 4.18, of these samples it is obvious that the amount of foam covering the particles decreases with increasing temperature. At 35◦ C, there are almost no visible particles seen using SEM. Our explanation for this is that when the hydrolysis rate of the TEOS is lower, the condensation of silica in the corona will be slow. During the hydrolysis in the core of the micelles, ethanol will be formed and which is a bad environment for the hydrophobic cores. The hydrophobic PO chains will repel the ethanol and in an ordinary synthesis the condensed silica will retain the shape of the micelle while the ethanol diffuses out of the micelle. But when the hydrolysis rate is slow, the small amounts of condensed silica will not be able to keep the shape of the micelle which then will be destroyed. When the reaction temperature is increased, the condensation of silica will be faster and will be able to retain the shape of the micelles which leads to an ordered material. Comparing the 50◦ C ethanol sample with the samples without additives, they look quite the same. There are very little foam and the hysteresis loops are very distinct type 1. The only difference between these samples is the maximum value of the pore size distribution where the sample without additives has a pore size of 6.4 nm and the sample containing ethanol has a pore size of 8.2 nm. It is clear that ethanol is working as a swelling agent, but that the reaction needs to be increased to 50◦ C to get a clean product. 5.4. CHANGING PH 5.4 59 Changing pH ME24-3M-PH1 and ME24-3M-PH2 do not have an ordered pore structure. The pore size distribution of ME24-3M-PH1 shows a small peak at 7.7±4 nm. This peak can be explained by the fact that there still are cylindrical pores even if they are not ordered in an hexagonal structure which can be seen in figure 4.24(a). This figure looks similar to the cryo-TEM images made by Ruthstein et al [20] who studied the formation of SBA-15. Even when the temperature is increased to 50◦ C in the case of methanol additions the reaction is too slow to form the final product in 24h. Instead the product resembles the state of an ordinary synthesis during the formation of the hexagonal structure in the reaction. When the pH is increased further to 2 we only see a foam in the TEM images, see figure 4.24(b). This looks like the cryo-TEM images of the development of threadlike micelles in the reaction for an ordinary sample. This confirms our theory that the increase in pH together with methanol decreases the reaction speed. 60 CHAPTER 5. DISCUSSION Chapter 6 Conclusions This study has showed that it is possible to control the pore size and order of SBA15 using swelling agents and changing synthesis parameters. Methanol, ethanol, isopropanol, formaldehyde and acetone have all proved to be acting as swelling agents in the synthesis. THF on the other hand is not a suitable swelling agent using the standard parameters when synthesizing SBA-15. It has been shown that the swelling agent/P123 ratio should be below 420 to get an ordered structure in the material. A ratio of 210 gives an increase in pore size from 6.4 to approximately 8.0±0.4 nm using alcohols, formaldehyde or acetone as swelling agent. It was further shown that swelling agents with a high dipole moment give a higher pore size than molecules with less dipole moment. Increasing the hydrothermal treatment time when alcohols are used as swelling agents does not increase the pore size, which is in opposite to treatment of samples without, or with alkenes as, swelling agents. However, an increase in time is increasing the order in the samples and reduces the amount of the foamy secondary phase, i.e increasing the yield . Increasing the hydrothermal treatment temperature increases the pore size and also the order in the samples The reaction temperature is a crucial parameter for making ordered products. When ethanol is used as a swelling agent, the reaction temperature needs to be increased to 50◦ C to get a clean sample without foam. When the reaction temperature is as low as 35◦ C, the material contains of a lot of foam and few particles with a hexagonally ordered pore structure. When the pH is increased in combination with methanol the reaction takes much longer time. The resulting products look like snapshots of the synthesis before the reaction is finished. 61 62 CHAPTER 6. CONCLUSIONS Chapter 7 Future works There are many parameters that should further be explored for deepening the understanding of pore size and structure. • Swelling agents with higher dipole moment than acetone should be explored to confirm the theory that an increase in dipole moment increases the pore size. • Study the effect of hydrothermal treatment for ketones as swelling agents. • Explore deeper the effect of hydrothermal treatment when alcohols are used as swelling agents. Especially the microporosity in these samples should be studied to confirm the hypothesis stated in the discussion. Further should synthesis be made with even longer hydrothermal treatment times be made to see if the pore size increases. • Investigate if larger changes in reaction temperature can give an ordered porosity when the amount of swelling agents are used and also what happens with the ethanol samples when the reaction temperature is increased further. Also applications of the material should be developed by introducing functional groups inside the pores. Attempts to use these materials as mesoreactors will be made. There are also ideas about using SBA-15 for hydrogen storage, but these ideas needs to be developed further. 63 64 CHAPTER 7. FUTURE WORKS Bibliography [1] C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, and J. S. Beck. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature, 359:710, 1992. [2] J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T-W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins, and J. L. Schlenker. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc, 114:10834, 1992. [3] K. S. W. Sing, D. H. Everett, R. A. W Haul, L. Moscou, R. A. Pierotti, J. Rouquérol, and T. Siemieniewska. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure & Appl. Chem., 57:603, 1985. [4] F. Schüth, K. S. W. Sing, and J. Weitkamp. Handbook of Porous Solids, volume 1. WILEY-VCH Verlag GmbH, 2002. [5] A. Sayari. Novel synthesis of high-quality MCM-48 silica. J. Am. Chem. Soc, 122:6504, 2000. [6] D. Zhao, Q. Huo, J. Feng, B. F. Chmelka, and G. D Stucky. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc, 120:6024, 1998. [7] D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka, and G. D Stucky. Triblock copolymer syntheses of mesoporous silica with peiodic 50 to 300 angstrom pores. Science, 279:548, 1998. [8] J. S. Lettow, Y. J. Han, P. Schmidt-Winkel, P. Yang, D. Zhao, G. D. Stucky, and J. Y. Ying. Hexagonal to mesocellular foam phase transition in polymertemplated mesoporous silicas. Langmuir, 16:8291, 2000. 65 66 BIBLIOGRAPHY [9] T. P. B. Nguyen, J.-W. Lee, W. G. Shim, and H. Moon. Synthesis of functionalized SBA-15 with ordered large pore size and its adsorption properties of BSA. Microporous and Mesoporous Materials, 110:560, 2008. [10] N. El-Hassan, E. Delahaye, V. Escax, P. Beaunier, M.-D. Appay, and A. Davidson. SBA-15 silicas as hard templates for the nanocasting of oxide nanoparticles. Annales de Chimie: Science des Materiaux, 30:315, 2005. [11] M. Kand, S. H. Yi, H. I. Lee, J. E. Yie, and J. M. Kim. Reversible replication between ordered mesoporous silica and mesoporous carbon. Chem. Comm., page 1944, 2002. [12] U.-H. Lee, M. h. Kim, and Y.-U. Kwon. Mesoporous thin films with accessible pores from surfaces. Bull. Korean Chem. Soc., 27:808, 2006. [13] R. Ryoo and C. H. Ko. Block-copolymer-templated ordered mesoporous silica: array of uniform mesopores or mesopore-micropore network? J. Phys. Chem. B, 104:11465, 2000. [14] M. Impéror-Clerc, P.Davidson, and A.Davidson. Existencs of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers. J. Am. Chem. Soc, 122:11925, 2000. [15] S. Ruthstein, V. Frydman, S. Kababya, M. Landau, and D. Goldfarb. Study of the formation of the mesoporous materials SBA-15 by EPR spectrocsopy. J. Phys. Chem. B, 107:1739, 2003. [16] L. Vradman, L. Titelman, and M. Herskowitz. Size effect on SBA-15 microporosity. Microporous and Mesoporous Materials, 93:313, 2006. [17] S. Ruthstein, V. Frydman, and D. Goldfarb. Study of the initial formation stages of the mesoporous material SBA-15 using spin-labeled block copolymer templates. J. Phys. Chem. B, 108:9016, 2004. [18] K. Flodström, H. Wennerström, and V. Alfredsson. Mechanism of mesoporous silica formation. a time-resolved nmr and tem studyy of silica-block copolymer aggregation. Langmuir, 20:680, 2004. [19] K. Flodström, C. Teixeira, H. Amenitsch, V. Alfredsson, and M. Lindén. In situ synchrotron small-angle X-ray scattering/X-ray diffraction study of the formation of SBA-15 mesoporous silica. Langmuir, 20:4885, 2004. [20] S. Ruthstein, J. Schmidt, E. Kesselman, Y. Talmon, and D. Goldfarb. Resolving intermediate solution structures during the formation of mesoporous SBA-15. J. Am. Chem. Soc, 128:3366, 2006. BIBLIOGRAPHY 67 [21] J. Liu, Q. Yang, X. S. Zhao, and L. Zhang. Pore size control of mesoporous silicas from mixtures of sodium silicate and teos. Microporous and Mesoporous Materials, 106:62, 2007. [22] J. Sun, H. Zhang, D. Ma, Y. Chen, X. Bao, A. Klein-Hoffman, N. Pfänder, and D. S. Su. Alkanes-assosted low temperature formation of highly ordered SBA-15 with large cylindrical mesopores. Chem. Comm., page 5343, 2005. [23] P. Schmidt-Winkel, W. W. Lukens, D. Zhao, P. Yang, B. F. Chmelka, and G. D. Stucky. Mesocellular siliceous foams with uniformly sized cells and windows. Microporous and Mesoporous Materials, 22:485, 1998. [24] Y.-L. Su and H.-Z. Liu. Temperature-dependent solubilization of PEO-PPOPEO block copolymers and their application for extraction trace organics from aqueous solutions. Korean J. Chem. Eng., 20:343, 2003. [25] D. F. Evans and H. Wennerström. The colloidal domain: where physics, chemistry, biology, and technology meet. VCH Publishers, 1994. [26] P. Kipemboi, A. Fogden, V. Alfredsson, and K. Flodström. Triblock copolymers as templates in mesoporous silica formation: Structural dependence on polymer chain length and synthesis temperature. Langmuir, 17:5398, 2001. [27] R. Aelion, A. Lobbel, and F. Eirich. Hydrolysis of ethyl silicate. J. Am. Chem. Soc, 72:5705, 1950. [28] T. Yamada, H. Zhou, K. Asai, and I. Honma. Pore size controlled mesoporous silicate powder prepared by triblock copolymer templates. Materials Letters, 56:93, 2002. [29] M. Kruk and L. Cao. Pore size tailoring in large-pore SBA-15 silica synthesized in the presence of hexane. Langmuir, 23:7247, 2007. [30] J. L. Blin and B. L. Su. Tailoring pore size of ordered mesoporous silicas using one or two organic auxiliaries as expanders. Langmuir, 18:5303, 2002. [31] A. G. Denkova, E. Mendes, and M.-O. Coppens. Effects of salts and ethanol on the population and morphology of triblock copolymer micelles in solution. J. Phys. Chem. B, 112:793, 2008. [32] T. N. M. Bernards, M. J. van Bommel, and A. H. Boonstra. Hydrolysiscondensation processes of the tetra-alkoxysilanes TPOS, TEOS and TMOS in some alcoholic solvents. J. Non-Cryst. Sol., 134:1, 1991. [33] P. F. Fulvio, S. Pikus, and M. Jaroniec. Short time synthesis of SBA-15 using various silica sources. J. Coll. Int. Sc., 287:717, 2005. 68 BIBLIOGRAPHY [34] P. F. Fulvio, S. Pikus, and M. Jaroniec. Tailoring properties of SBA-15 materials by controlling conditions of hydrothermal synthesis. J. Mat. Chem., 15:5049, 2005. [35] J. M. Kim and G. D. Stucky. Synthesis of highly ordered mesoporous silica materials using sodium silicate and amphiphilic clock copolymers. Chem. Comm., page 1159, 2000. [36] D. Zhao, J. Sun, Q. Li, and G. D. Stucky. Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater., 12:275, 2000. [37] C. Yu, B. Tian, J. Fan, G. D. Stucky, and D. Zhao. Salt effect in the synthesis of mesoporous silica templated by non-ionic block copolymers. Chem. Comm., page 2726, 2001. [38] C. Booth and D. Attwood. Effects of block architecture and composition on the association properties of poly(oxyalkylene) copolymers in aqueous solution. Mater. Chem. Phys., 108:73, 2008. [39] P. Schmidt-Winkel, P. Yang, D. I Margolese, B. F. Chmelka, and G. D. Stucky. Fluoride-induced hierarchial ordering of mesoporous silica in aqueous acid-synthesis. Adv. Mater., 11:303, 1999. [40] N. Brodie-Linder, G. Dosseh, C. Alba-Simonesco, F. Audonnet, and M. Impéror-Clerc. SBA-15 synthesis: Are there lasting effects of tmeperature change within the first 10 min of TEOS polymerization? Macromol. Rapid Commun., 21:501, 2000. [41] E. B. Celer, M. Kruk, Y. Zuzek, and M. Jaroniec. Hydrothermal stability of SBA-15 and related ordered mesoporous silicas with plugged pores. J. Mat. Chem., page 2824, 2006. [42] A. Galarneau, H. Cambon, F. Di Renzo, and F. Faluja. True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature. Langmuir, 17:8328, 2001. [43] J. C. P. Broekhoff and J. H. De Boer. Studies on pore systems in catalysts : X. calculations of pore distributions from the adsorption branch of nitrogen sorption isotherms in the case of open cylindrical pores b. applications. J. Catal, 9:15, 1967. [44] M. Kruk, M. Jaroniec, C. H. Ko, and R. Roo. Characterization of the porous structure of SBA-15. Chem. Mater., 12:1961, 2000. BIBLIOGRAPHY 69 [45] M. Choi, W. Heo, F. Kleitz, and R. Roo. Facile sythesis of high quality mesoporous SBA-15 with enhanced control of the porous network connectivity and wall thickness. Chem. Comm., page 1340, 2003. [46] S. Brunauer, P. H. Emmett, and E. Teller. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc, 60:309, 1938. [47] M. Kruk, M. Jaroniec, and A. Sayari. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir, 13:6267, 1997. [48] M. Jaroniec and L. A. Solovyov. Improvement of the Kruk-Jaroniec-Sayari method for pore size analysis of ordered silicas with cylindrical mesopores. Langmuir, 22:6757, 2006. [49] Q. Hu, H. Suzuki, H. Gao, H. Araki, W. Yang, and T. Noda. High-frequence ftir absorption of SiO2 /Si nanowires. Chem. Phys. Lett., 378:299, 2003. [50] B. Tian, X. Liu, C. Yu, F. Gao, S. Xie, B. Tu, and D. Zhao. Microwave assisted template removal of siliceous porous materials. Chem. Comm., page 1186, 2002. 70 BIBLIOGRAPHY Appendix A Samples Table A.1: Results for all samples. Sample ME24 ME72 ME24-3M ME72-3M ME24-3M-PH1 ME24-3M-PH2 ME24-3M-125A ME24-3M-140A ME24-6M ME24-3E ME24-3E-35R ME24-3E-45R ME24-3E-50R ME72-3E ME72-3E-45R-125A ME72-3E-45R-140A ME24-6E ME24-6E-35R ME24-6E-45R ME24-3I ME72-3I ME24-6I ME24-3F ME24-3A ME24-6A ME24-3T d(100) spacing [nm] 8.8 9.0 9.9 8.1 8.7 8.5 8.2 8.3 8.1 8.0 9.4 9.6 9.0 8.3 8.2 7.8 8.6 8.3 8.4 9.1 8.5 8.5 8.2 71 Pore size [nm] 6.4 7.2 8.2 8.2 7.8 8.2 9.6 7.3 8.2 7.3 8.2 8.2 8.2 8.2 9.6 1.5 7.3 7.3 7.3-8.0 8.2 8.2 8.0 8.5 1.5 7.5 Wall thickness [nm] 3.8 3.2 3.2 1.2 1.8 2 2.3 1.3 2.1 1.0 2.7 2.9 22 0 8.2 1.7 2.6 1.9 1.5 2.5 1.3 8.2 2.0