Experimental procedure and Figs. S1 - S4
advertisement
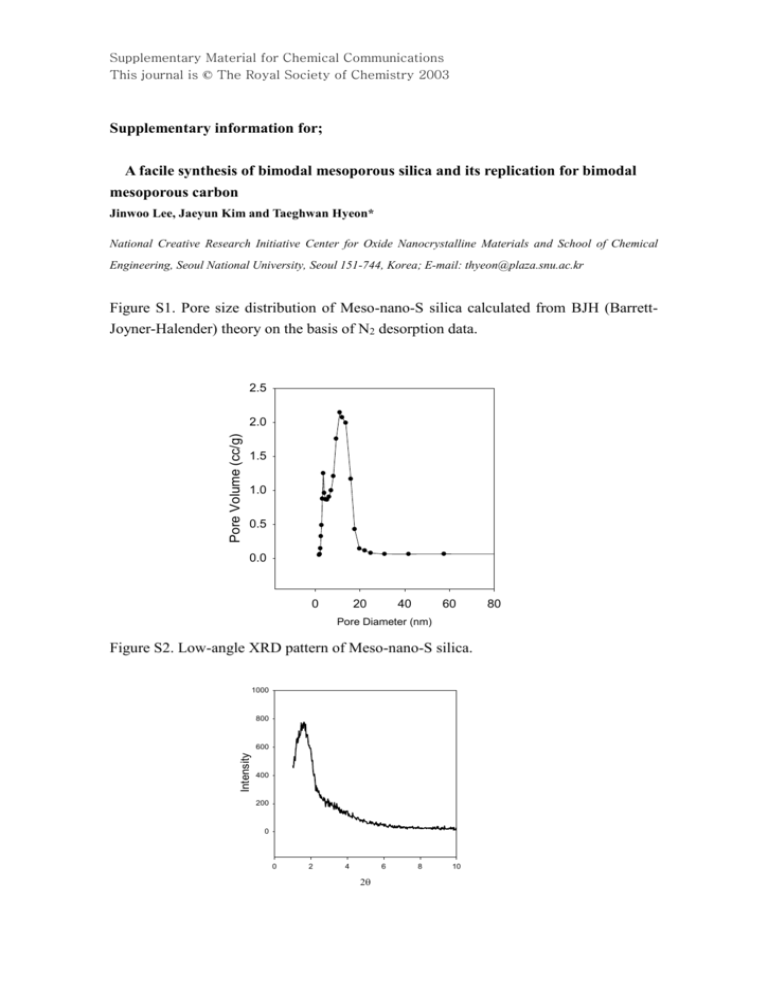
Supplementary Material for Chemical Communications This journal is © The Royal Society of Chemistry 2003 Supplementary information for; A facile synthesis of bimodal mesoporous silica and its replication for bimodal mesoporous carbon Jinwoo Lee, Jaeyun Kim and Taeghwan Hyeon* National Creative Research Initiative Center for Oxide Nanocrystalline Materials and School of Chemical Engineering, Seoul National University, Seoul 151-744, Korea; E-mail: thyeon@plaza.snu.ac.kr Figure S1. Pore size distribution of Meso-nano-S silica calculated from BJH (BarrettJoyner-Halender) theory on the basis of N2 desorption data. 2.5 Pore Volume (cc/g) 2.0 1.5 1.0 0.5 0.0 0 20 40 60 Pore Diameter (nm) Figure S2. Low-angle XRD pattern of Meso-nano-S silica. 1000 800 Intensity 600 400 200 0 0 2 4 6 8 10 80 Supplementary Material for Chemical Communications This journal is © The Royal Society of Chemistry 2003 Figure S3. Low-angle XRD pattern of Meso-nano-C carbon (dspacings= 4.66 nm). 1400 1200 1000 Intensity 800 600 400 200 0 0 2 4 6 8 10 2 Figure S4. Nitrogen adsorption-desorption isotherms for the Mesoporous carbon prepared using 0.7cc phenol per g of Meso-nano-S. Inset: Corresponding pore size distribution calculated from BJH (Barrett-Joyner-Halender) theory on the basis of N2 adsorption data. The average pore size and surface area of this unimodal mesoporous carbon are 4.24 nm and 1051 m2/g, respectively. 800 Adsorption Desorption 700 500 4 400 Pore volume (cc/g) Volume Adsorbed (cc/g) 600 300 200 3 2 1 100 0 0 5 10 15 20 25 30 35 40 45 50 55 60 Pore Diameter(nm) 0 0.0 0.2 0.4 0.6 Relative Pressure (P/Po) 0.8 1.0 Supplementary Material for Chemical Communications This journal is © The Royal Society of Chemistry 2003 Text S1. Experimental procedure for the Synthesis of Meso-nano-C 1 g sample of as-synthesized Meso-nano-S was dispersed in 20 ml of 95% ethanol containing 1 ml of concentrated HCl solution, in order to extract the surfactant and sodium ions. After the extraction was accomplished by means of stirring, the powder was retrieved by filtration and dried in an oven at 100 oC for 12 h. Then, 1 g of dried Meso-nano-S silica was dispersed in 5 ml of H2O containing 0.2 g of AlCl3·6H2O and the resulting mixture was stirred for 30 min at room temperature to generate acidic aluminum sites. After complete drying in an oven at 80 oC, the precipitate was calcined in air at 550 oC, in order to obtain the Meso-nano-S aluminosilicate. The desired amount of phenol was infiltrated into the mesopores of Meso-nano-S aluminosilicate by heating at 100 oC under a static vacuum. The resulting phenol/Meso-nano-S aluminosilicate composite and excess para-formaldehyde were reacted in an autoclave at 140 oC for 24 h. The composite was heated at 1 oC /min to 140 oC and held at this temperature for 5 h under an argon atmosphere. Afterwards, the composite was pyrolyzed by heating at 800 oC for 3 h under a nitrogen atmosphere. The dissolution of the Meso-nano-S aluminosilicate template, using 5 wt% HF solution diluted with 50% ethanol and 50% H 2O, generated mesoporous carbon Mesonano-C.