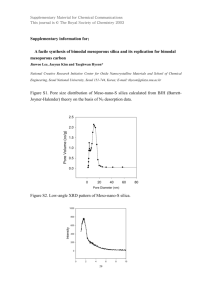
Synthesis and characterisation of mesoporous silica foam based
advertisement

Synthesis and characterisation of mesoporous silica foam based titanate nanostructure composites Dorina Dobó1,2, Tamás Somogyi1, Dr. Ákos Kukovecz1,2, Dr. Zoltán Kónya1,3 1 University of Szeged, Department of Applied and Environmental Chemistry, Szeged 2 MTA-SZTE "Lendület" Porous Nanocomposites Research Group, Szeged 3 MTA-SZTE Reaction Kinetics and Surface Chemistry Research Group, Szeged Nowadays, several materials science studies are focused on nanoscaled materials due to their unique properties (electronic, thermal, mechanical, magnetic, etc.) related to their tiny size which makes these materials different from their bulk counterparts. Titanate nanotubes were produced first by Kasugaet al in 1999 from titanium dioxide (TiO2). Later, several different preparation techniques have been developed and titanate nanowires (TiONW) were also successfully synthesized by Horvath et al. Synthesis, characterisation and application of different porous solids have long been popular in the area of material science. Porous materials have high specific surface area thus they can be used as adsorbents, shape selective Fig.1. TEM image of mesocellular silica foam based TiONW catalysts and support for catalyst. Porous materials can be divided in three groups in function of their pore size: microporous- have a pore diameter (PD) less than 2 nm; mesoporous PD 2-50 nm, and the macroporous materials PD>50 nm. Macrocellular mesoporous silicate foams were prepared by a modified sol-gel route based on the technique suggested by Bagshaw. In this work, we have synthesized mesoporous Fig.2. SEM image of mesocellular silica foam based TiONW silica - TiONW composites. Our aim was to reproducibly synthesise layered nanocomposites that have a high specific surface area and small pore diameter. The as-prepared materials were characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM), optical microscopy, X-ray diffractometry (XRD), thermogravimetry (TG/DTA) and specific surface areas were determined from the N2 adsorption isotherms (BET).